Production of biosurfactant by a Pseudomonas aeruginosa isolate and its applicability to in situ microbial enhanced oil recovery under anoxic conditions
Feng Zhaoa,
Jie Zhanga,
Rongjiu Shib,
Siqin Hanb,
Fang Ma*a and
Ying Zhang*b
aState Key Laboratory of Urban Water Resource and Environment, School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin 150090, China. E-mail: mafanghitzf@126.com
bKey Laboratory of Pollution Ecology and Environmental Engineering, Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, China. E-mail: yzhang207@126.com
First published on 15th April 2015
Abstract
Compared to ex situ application, in situ application of biosurfactants for microbial enhanced oil recovery (MEOR) is relatively cost-effective, and lack of oxygen in oil reservoirs is a bottleneck for in situ production of biosurfactants by mostly isolated biosurfactant-producing bacteria. Furthermore, few microorganisms can produce biosurfactants under anoxic conditions. A bacterial strain identified as Pseudomonas aeruginosa SG (GenBank accession number KJ995745) was isolated from Xinjiang oil field, and it can produce biosurfactant under anoxic conditions. Different organic substrates (glucose, sucrose, glycerol, corn steep powder, starch, molasses, soybean oil, sunflower oil) were tested to determine the optimal carbon source for anoxic production of biosurfactant by SG. Strain SG anaerobically grew well at temperatures (25–40 °C), pH (6.0–9.0), and salinity (0–30 g L−1 of NaCl), respectively. Thin layer chromatography and Fourier transform infrared spectroscopy revealed that the SG biosurfactant produced under anoxic conditions was similar to rhamnolipid. SG biosurfactant could reduce air–water surface tension from 71.6 to 33.3 mN m−1, and reduce oil–water interfacial tension from 26.1 to 2.14 mN m−1. And a critical micelle concentration value of 80 mg L−1 was obtained. Moreover, the biosurfactant displayed good emulsifying activity over hydrocarbons and crude oil. A core flooding test revealed that an extra 8.33% of original crude oil in the core was displaced through the in situ production of rhamnolipid by SG. The potential use of the isolated SG for in situ MEOR application was discussed. And bioaugmentation of SG in Xinjiang oil reservoirs will be a promising approach for in situ MEOR.
Introduction
Biosurfactants are a series of surface-active substances produced by microorganisms.1 As natural potent oil-displacing agents, biosurfactants were applied in microbial enhanced oil recovery (MEOR) due to their biodegradability, ecological safety, high surface activity, and low critical micelle concentration (CMC).2–5 Microbial enhanced oil recovery (MEOR) technologies utilize microbial metabolites such as biosurfactants to emulsify crude oil and to lower oil–brine interfacial tension, and hence mobilize entrapped oil.6–8 In the MEOR process, alteration of oil/water/rock interfacial properties by the biosurfactant product of microorganisms leads to enhanced oil recovery.9 Biosurfactant flooding as a green and promising enhanced oil recovery technology has been extensively studied.10,11Previous studies of MEOR were mostly concentrated on the screening of aerobic functional microorganisms and the evaluation their potential of enhanced oil recovery under aerobic conditions.12 However, the ex situ application of biosurfactants to enhance oil recovery is costly and complex in terms of biosurfactants production and transportation. In situ application of biosurfactants is considered to be more advantageous for MEOR.13,14 Due to lack of oxygen in oil reservoirs, the growth and metabolic process of isolated aerobic biosurfactant-producing microorganisms in oil reservoirs was significantly restricted. In the actual application process, the right amount of air was pumped into oil reservoirs for MEOR technology based on aerobic microorganisms, but pumping of air is costly, poor operation and low security.
Therefore, microorganisms that can produce biosurfactants under anoxic conditions are urgently needed. High-throughput sequencing data showed that there are abundant microbial species resources in oil reservoirs. In addition, studies have reported that the microbial strains can be used as inocula actually grow and metabolize in the oil reservoirs.6,15 It is indispensible for in situ MEOR application to screening anaerobic or facultative anaerobic biosurfactant-producing microorganisms from oil reservoirs. However, information on production of biosurfactant under anoxic conditions is scarce.16,17 In a previous study, we reported construction an engineered bacterial strain and evaluate its anaerobic production of biosurfactant for microbial enhanced oil recovery (MEOR).18 Although the strain achieved anaerobic and heterologous production of rhamnolipid biosurfactant and had great potential for MEOR, engineered strain may have unstable function and cause environmental risk when used it in oil reservoirs. Therefore, isolating bacterial strains that can produce biosurfactant under anoxic conditions from oil reservoirs is a promising approach to overcome the limits of oxygen-depleted environments for in situ MEOR application.
In the present study, a bacterial strain which can produce biosurfactant under anoxic conditions was isolated from Xinjiang oil field, China. And its metabolic properties, biosurfactant activity and potential of enhanced oil recovery were evaluated under anoxic conditions. The results of this study will contribute to the optimization of MEOR processes to overcome the anoxic environments in oil reservoirs.
Results and discussion
Isolation and characterization of strain SG
Through the enrichment method, fifteen strains which could reduce the surface tension of culture broth to lower than 35 mN m−1 were isolated. After incubating the strains under anoxic conditions, only one bacterial strain, designated SG, could significantly reduce the surface tension of anoxic culture broth (from 63.4 mN m−1 to 33.3 mN m−1). Morphological analysis showed that the SG strain was Gram-negative, non-spore-forming, rod shaped, and the colonies of SG were fold surface, jagged edge, producing green pigment. The scanning electron microscope (SEM) photograph of SG strain was shown in Fig. 1.The 16S rRNA gene sequence of SG strain was deposited in the GenBank database under accession number KJ995745. Multiple alignments showed that 16S rDNA sequence of SG was very similar to that of Pseudomonas aeruginosa VRFPA04 (99% similarity, CP008739) and P. aeruginosa PA96 (99% similarity, CP007224). The approximate phylogenetic position of strain SG is shown in Fig. 2. Based on morphological characterization and the phylogenetic analysis of 16S rRNA gene sequence, the isolated strain SG was identified as a strain of P. aeruginosa, and named as P. aeruginosa SG. This strain P. aeruginosa SG was isolated from oil reservoirs. Bioaugmentation this indigenous strain SG in oil reservoirs is more advantageous for in situ MEOR than the engineered bacterial strain reported in previous study,18 in terms of stably producing biosurfactant under anoxic conditions and the low environmental risk in oil reservoirs.
Environmental adaptability of strain SG
Effects of temperatures, pH and salinity on strain SG were investigated. Growth was defined as reducing the surface tension of culture broth to lower than 35 mN m−1 within 7 days. Growth of SG occurred over a temperature range of 25 °C to 40 °C, a pH range from 6.0 to 9.0, and a salinity range from 0% to 3% NaCl. Result showed that strain SG has a relatively wide scope of environmental adaptability. Strain SG can grow and produce biosurfactant under medium temperature (25 °C to 40 °C), weak alkaline environment, high concentration of salinity (as high as 30 g L−1 NaCl), which gives a potential for its application of enhance oil recovery.Optimal carbon source and nitrogen source for strain SG under anoxic conditions
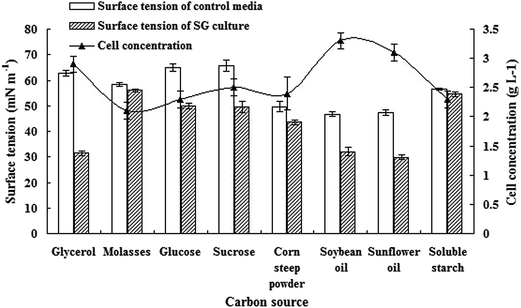
Carbon source and nitrogen source are significant factors for biosurfactant production. Wu et al. (2008) reported that NaNO3 was the best nitrogen source for biosurfactant production.19 Furthermore, NaNO3 can be used as electron accepter when strain grew under anoxic conditions. Therefore, in this study, NaNO3 was selected as the best nitrogen source for anaerobic production of biosurfactant by SG. Besides, rhamnolipid biosurfactant is mixed congeners in various proportions, including one or two rhamnoses attached to different lengths of β-hydroxy fatty acid chains.20 Using different carbon sources, microorganisms may produce rhamnolipid congeners with different structures and different proportions, which lead to the biosurfactant product possessing different surface activities. Surface activity is an important parameter for biosurfactant. Fig. 3 lists the carbon source tests. Among the examined carbon sources, glycerol exhibited maximum surface tension reduction extent (decreased from 63.40 mN m−1 to 32.63 mN m−1), followed by sunflower oil (decreased from 47.4 mN m−1 to 29.8 mN m−1), soybean oil (decreased from 46.7 mN m−1 to 32.0 mN m−1). Moreover, glycerol is soluble in water and can be easily absorbed and metabolized by microorganisms.21 As a major byproduct of biodiesel, glycerol is also a promising and inexpensive carbon source for production of biosurfactants.22,23 Hence, glycerol was chosen as carbon source for biosurfactant production by SG under anoxic conditions.Preliminary characterization of biosurfactant
As shown in Fig. 4A, thin layer chromatography (TLC) analysis revealed that the SG biosurfactant product is similar to the rhamnolipid product of strain WJ-1.24 The FT-IR spectrum of biosurfactant produced by SG was shown in Fig. 4B. The characteristic absorption bands of biosurfactant were the absorption bands around 2918, 2849, and 1462 cm−1 caused by the symmetric C–H stretching vibrations of aliphatic groups and the 1712 cm−1 absorption band caused by the presence of ester groups. In the fingerprint region of the spectrum, the absorption area between 1462 and 1060 cm−1 represents C–H and O–H deformation vibrations, which are typical for carbohydrates. The FT-IR spectrum of SG biosurfactant is similar to the reported spectra of rhamnolipid.17,24,25 Through TLC and FT-IR spectra analyses, the biosurfactant produced by SG under anoxic conditions was preliminarily characterized as rhamnolipid.The surface tension of SG biosurfactant solutions rapidly decreased with the increase of concentration until the surface tension reached the minimum (33.2 mN m−1). Then the surface tension values remained constant with the increase of concentration. At the turning point, the biosurfactant concentration, i.e., CMC, is 80 mg L−1. While the CMC of biosurfactant produced by the engineered bacterial strain reported in our previous study18 is 90 mg L−1, which is possibly attributed to the different structures of the produced biosurfactant. CMC represents the surface activity of surfactants. The lower the CMC of one surfactant is, the lower the concentration for this surfactant to form micelle. And the chemical surfactants sodium dodecyl sulfate (SDS) has a CMC value of 2100 mg L−1.26
Biosurfactant stability
The SG biosurfactant stability was studied according to the surface tension of SG cell-free culture supernatant under different temperatures, pH, and salinity. The surface tension of SG culture supernatant maintains about 32.2 mN m−1 at a temperature range from 4 °C to 121 °C; and the surface tension is lower than 34.7 mN m−1 in pH ranging from 2 to 10; and the surface tension is lower than 34.5 mN m−1 in NaCl concentration less than 15%. Results indicate that the biosurfactant produced by SG is well thermostable, salt-tolerant, and well acidophilic. These properties make the SG biosurfactant potentially adapt to the complex and extreme environments.Interfacial activity
Biosurfactant product of SG could reduce oil–water interfacial tension (IFT) from 26.1 mN m−1 to 2.14 mN m−1. Biosurfactants could reduce oil–water IFT, thus decreasing the energy required to mobilize trapped oil in oil reservoir, and displace oil to a production well.27 IFT reduction by 2–3 orders of magnitude is generally required for successful displacement of residual oil.28 However, Wang et al. (2007) constructed an engineered strain P. aeruginosa PEER02 that its rhamnolipid product can reduce IFT by up to one order of magnitude, it was still shown to have potential for enhanced oil recovery application (recovered 42% of trapped oil in a sand pack).29 The SG biosurfactant product in this study also could significantly reduce the oil–water IFT, so it is promising to mobilize trapped oil in oil reservoirs.Emulsification activity
The SG cell-free culture showed good emulsifying activity to all the tested hydrophobic organics (all EI24 > 56%), and can emulsify crude oil up to EI24 ≈ 80%. The good emulsifying activity of the SG biosurfactant makes it potentially to enhance oil recovery. Biosurfactants aid oil emulsification and can contribute to the detachment of oil films from rocks,29 which will be beneficial to mobilize trapped oil in subsurface oil reservoirs to enhancing oil recovery.Biosurfactant production in anaerobic fermentor
Fig. 5 shows the pattern of biosurfactant formation, nitrate consumption and cell growth of P. aeruginoasa SG in the anaerobic GN medium. Optical density of OD600 was used to monitor cell growth. The surface tension of the culture medium decreased from 63.4 mN m−1 to 34.5 mN m−1 in 24 h. SG growth entered into stationary phase from 34 h, and maximum cell concentration of 1.912 (OD600) was obtained at 58 h. In Fig. 5, nitrate was constantly consumed during cell growth and biosurfactant production. At first, strain SG performs anaerobic respiration using nitrate as an electron acceptor, and then starts endogenous respiration when nitrate depleted at 70 h. And there is a fast biosurfactant formation rate in N-limitation condition (from 34 h to 142 h). Limiting nitrogen source promotes biosurfactant biosynthesis.19,20 The highest yield of biosurfactant (1.08 g L−1) was occurred at 142 h in anaerobic fermentation process. In a previous study, we constructed an engineered strain P. stutzeri Rhl that can produce 1.61 g L−1 biosurfactant under anoxic conditions.18 In this study, the isolated strain P. aeruginosa SG can produce 1.08 g L−1 of biosurfactant under anoxic conditions. This may result from the great complexity of quorum-sensing and the transcriptional regulatory network involved in rhamnolipids biosynthesis in P. aeruginosa.18,30 And the minimum concentration of biosurfactant required to mobilize the entrapped oil from sandstone cores is about 10 mg L−1.13,14 Results demonstrated the potential feasibility of strain P. aeruginosa SG for MEOR through in situ production of biosurfactant.Enhanced oil recovery in core flooding test under anoxic conditions
Core flooding test was conducted to evaluate the enhanced oil recovery efficiency of SG. Due to the volumetric sweep efficiency, the first water flooding resulted in 55.61% of the oil recovered from the core. At the end of the second water flooding, 63.94% of oil was recovered. P. aeruginosa SG could produce rhamnolipid under anoxic conditions, which can contribute to mobilize entrapped oil in core model through in situ production of rhamnolipid. The enhanced oil recovery efficiency of strain SG in core model is 8.33%, which suggested that the bacterial strain P. aeruginosa SG has a great potential for in situ MEOR applications.Bioaugmentation of strain SG in oil reservoirs will be a promising approach for in situ MEOR. Bioaugmentation of biosurfactants-producing microorganisms into oil reservoirs has been generally acknowledged to be a relatively cost-effective approach comparing to injection of biosurfactants products.13,14 Efficiently producing biosurfactants under oxygen limiting conditions is crucial for in situ MEOR applications. Future research would concentrate on optimization the rhamnolipid yield of SG under anoxic conditions.
Conclusion
In the current work, the biosurfactant production under anoxic conditions by the isolate, P. aeruginosa SG, was investigated. The growth parameters of SG were studied. Glycerol was chosen as the best carbon source for biosurfactant production by SG under anoxic conditions. Anaerobic production of biosurfactant by SG was confirmed in the anaerobic fermentor. Through TLC and FT-IR analysis, the surface active product was similar to rhamnolipid. The SG biosurfactant exhibits good surface-activity and emulsification properties. The enhanced oil recovery efficiency of strain SG in core flooding model is 8.33%, thus suggesting its interest for use in MEOR processes.Materials and methods
Chemicals and media
All the chemicals used for the experiments were of analytical grade. The mineral salts (MS) medium used for screening biosurfactant-producing microorganisms contained (per liter) 20 g glucose; 5.0 g NaNO3; 1.0 g KCl; 1.0 g NaCl; 4.4 g K2HPO4·3H2O; 3.4 g KH2PO4; 0.50 g MgSO4·7H2O; 0.5 g yeast extract. The pH of MS medium was adjusted to 7.0. The glycerol–nitrate (GN) medium31 was used for anoxic production of biosurfactant. Resazurin (final concentration, 0.0001% (wt per vol)) was added to verify the anaerobic medium was obtained. The anoxic cultivation experiments were conducted in serum bottles (250 ml) sealed with butyl rubber stoppers and caps.Isolation and characterization of facultative anaerobic biosurfactant-producing strain
Water samples were collected from Xinjiang oilfield, China. An enrichment method was used. Briefly, 10 mL of oilfield-produced water was added to the 500 mL flask containing 120 mL of MS medium and incubated at 37 °C, 180 rpm for 7 days. Then, 10 mL of the enrichment culture was transferred into fresh medium. Then, samples (0.1 mL) of serially diluted enrichment culture were dispersed on MS medium agar plates with 5% fresh sterile skimmed sheep blood. Each distinct colonies formed clear zone on the blood agar plate were inoculated into 150 mL flask containing 40 mL of MS medium and incubated at 37 °C, 180 rpm for 3 days. Then, cell-free culture supernatant (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min) was analyzed for surface tension by a BZY-1 automatic surface tension meter (Shanghai equitable Instruments Factory, China). The strains which can significantly reduce the surface tension of aerobic culture broth were inoculated into 250 mL serum bottles containing 200 mL of GN medium to evaluate their anaerobic production of biosurfactant. After 10 day incubation (80 rpm, at 37 °C), the candidate strain which can significantly reduce the surface tension of anaerobic culture broth was selected for further identification.
000 rpm, 10 min) was analyzed for surface tension by a BZY-1 automatic surface tension meter (Shanghai equitable Instruments Factory, China). The strains which can significantly reduce the surface tension of aerobic culture broth were inoculated into 250 mL serum bottles containing 200 mL of GN medium to evaluate their anaerobic production of biosurfactant. After 10 day incubation (80 rpm, at 37 °C), the candidate strain which can significantly reduce the surface tension of anaerobic culture broth was selected for further identification.
The morphological characterization of selected strain, referred to as strain SG, was determined from photomicrographs using scanning electron microscope (ESEM) Quanta™250 (FEI Company, American). The 16S rRNA gene of strain SG was amplified by PCR using universal primer pair 27F (5-AGAGTTTGATCCTGGCTCAG-3) and 1492R (5-GGTTACCTTGTTACGACTT-3). The PCR product was T-A cloned into plasmid pMD18 T and sequenced by Beijing Genomics Institution (Beijing, China), and the gene sequence was compared with those available in the GenBank database using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). In order to conduct phylogenetic relationships and cladistic analysis, a phylogenetic tree was constructed by the neighbor-joining method using MEGA 5.0 software. The topology of the phylogenetic tree was evaluated by 1000 bootstrap resampling replicates.32
Environmental adaptability of strain SG
Effects of temperature (10 °C, 20 °C, 25 °C, 30 °C, 37 °C, 40 °C, 45 °C), pH (5.0, 6.0, 6.5, 7.0, 7.5, 8.0, 9.0, 10.0) and salinity (NaCl concentration 0%, 1%, 3%, 5%, 7%, 9%, 11%) on strain SG growth was studied in 250 mL serum bottles containing 200 mL of GN medium. Growth was defined as reducing the surface tension of culture broth to lower than 35 mN m−1 within 7 days. Tests without inoculating strain SG were set as controls. All tests were run in triplicate.Selection of carbon source for strain SG
The isolated strain SG was cultivated in the anaerobically prepared, sterilized GN medium with equal amount of different organic substrates to determine the optimal biosurfactant anaerobic production. The 250 mL serum bottles were incubated on a rotary shaker at 80 rpm and 37 °C. Cell concentration and surface tension of culture media were measured after incubation. The tested organic substrates included glycerol, corn steep powder, carbohydrate (molasses, glucose, sucrose, soluble starch), and organic fatty acid (soybean oil and sunflower oil).Biosurfactant extraction and characterization
The biosurfactant was extracted by chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (v/v, 2
methanol (v/v, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).24,26 The extracted biosurfactant product was separated, visualized by thin layer chromatography (TLC) on silica gel G plates, and rhamnolipid product of P. aeruginosa WJ-124 was used as control. A 10 μL of biosurfactant sample (200 mg L−1) was placed on the silica gel G plates. After drying at room temperature, the chromatograms were developed with chloroform
1).24,26 The extracted biosurfactant product was separated, visualized by thin layer chromatography (TLC) on silica gel G plates, and rhamnolipid product of P. aeruginosa WJ-124 was used as control. A 10 μL of biosurfactant sample (200 mg L−1) was placed on the silica gel G plates. After drying at room temperature, the chromatograms were developed with chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol
methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (v/v/v, 90
water (v/v/v, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and visualized with a sulfuric acid–phenol TLC reagent at 95 °C for 10 min. The Fourier transform infrared (FT-IR) spectrum of biosurfactant product was recorded by a NICOLET 380 FT-IR spectrometer with a resolution of 0.5 cm−1 and frequency range of 400 cm−1 to 4000 cm−1. Furthermore, 10 mg of freeze-dried biosurfactant was mixed with 100 mg of KBr and pressed with 25 Mpa for 30 s to obtain translucent pellets for FT-IR spectra analysis. For determination of critical micelle concentration (CMC), SG biosurfactant solutions (concentrations of 0–120 mg L−1) were prepared, and the surface tension of the solutions was measured. Then, the surface tension–biosurfactant concentration curve was prepared. The surface tension of biosurfactant solution reaches the lowest at its CMC.
2) and visualized with a sulfuric acid–phenol TLC reagent at 95 °C for 10 min. The Fourier transform infrared (FT-IR) spectrum of biosurfactant product was recorded by a NICOLET 380 FT-IR spectrometer with a resolution of 0.5 cm−1 and frequency range of 400 cm−1 to 4000 cm−1. Furthermore, 10 mg of freeze-dried biosurfactant was mixed with 100 mg of KBr and pressed with 25 Mpa for 30 s to obtain translucent pellets for FT-IR spectra analysis. For determination of critical micelle concentration (CMC), SG biosurfactant solutions (concentrations of 0–120 mg L−1) were prepared, and the surface tension of the solutions was measured. Then, the surface tension–biosurfactant concentration curve was prepared. The surface tension of biosurfactant solution reaches the lowest at its CMC.
Interfacial tension and emulsification activity determination
The oil–water interfacial tension (IFT) of culture supernatant was measured by TX-500C interfacial tension meter. Emulsification index (EI24) was measured to evaluate emulsifying activity of SG biosurfactant product. Briefly, 4 mL of hydrophobic organics was mixed with 4 mL of SG culture supernatant in test tubes, vigorously stirred for 2 min and left to stand for 24 h. The emulsion index (EI24) (%) is defined as the height of the emulsion layer (mm) divided by the total height of the mixture (mm) and multiplied by 100.33 The tested hydrophobic organics included petroleum ether, kerosene, liquid paraffin, and crude oil (Xinjiang oilfield).Study of biosurfactant stability
Biosurfactant stability studies were carried out using the SG strain culture supernatant (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min). Briefly, the first batch of 20 mL of culture supernatant was treated at different temperatures (i.e., 4, 25, 35, 45, 60, 80, 100 and 121 °C) for 1 h and restored to room temperature; the pH of the second batch of 20 mL of culture supernatant was adjusted to different values (i.e., 2, 4, 6, 8, 10 and 12) with 1 M HCl and 1 M NaOH; the third batch of 20 mL of culture supernatant was treated at different salinity (i.e., NaCl concentration of 0%, 3%, 6%, 9%, 12%, 15%, 18%, 21% and 25%). The surface tension of the treated samples was measured to evaluate the biosurfactant stability.
000 rpm, 10 min). Briefly, the first batch of 20 mL of culture supernatant was treated at different temperatures (i.e., 4, 25, 35, 45, 60, 80, 100 and 121 °C) for 1 h and restored to room temperature; the pH of the second batch of 20 mL of culture supernatant was adjusted to different values (i.e., 2, 4, 6, 8, 10 and 12) with 1 M HCl and 1 M NaOH; the third batch of 20 mL of culture supernatant was treated at different salinity (i.e., NaCl concentration of 0%, 3%, 6%, 9%, 12%, 15%, 18%, 21% and 25%). The surface tension of the treated samples was measured to evaluate the biosurfactant stability.
Growth and biosurfactant production kinetics of SG in anaerobic fermentor
To investigate the time course of bacterial growth and biosurfactant production under anoxic conditions, cultivation test in a 6 L anaerobic fermentor (FerMac 310/60, Electrolab Biotech, UK) was conducted. The SG logarithmic phase culture was used as inoculum (6%, v/v) for anaerobic fermentation. The anaerobic GN medium was prepared as previously, and then sterilized at 121 °C for 20 min. The pH meter and ORP meter were used to determine the pH and oxidation–reduction potential (ORP) of the anaerobic culture. The initial pH was approximately 6.8. The pH value changed little during the tests and no pH adjustment was performed. Also, temperature probe and DO probe were equipped to the fermentor. The cell growth was carried out at 37 °C at 150 rpm for 214 hours with seal cultivation and no ventilation. Samples of culture were analyzed periodically for surface tension, rhamnolipid,29 nitrate, and cell concentration (OD600).Core flooding test under anoxic conditions
To evaluate the potential application of the strain SG in enhanced oil recovery, the core flooding test was employed. A standard core flooding equipment used was similar to that described before.24,34 The test was performed at 39 °C simulated the oil reservoir zone temperature at Xinjiang oilfield. The core is 291 mm in length, 38 mm in diameter and absolute permeability of 0.362 μm2. The core was saturated with formation water of Xinjiang oilfield after vacuum pumping. Then, the core was saturated with crude oil of Xinjiang oilfield (density of 0.886 g cm−3 and viscosity of 5.6 mPa s). After aging at 39 °C for 24 h, the core was flooded with formation water until no oil flowed out. After the first water flooding, 1 PV of culture solution (the cell precipitation of SG seed culture mixed with its anaerobic medium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v)) was injected into core model. The core was then incubated at 39 °C for 8 d. The core was flooded again with the same formation water. The flow rate for the flooding was set at 0.2 mL min−1. The amount of displaced oil (mL) and displaced water (mL) in core flooding process were recorded.
20, v/v)) was injected into core model. The core was then incubated at 39 °C for 8 d. The core was flooded again with the same formation water. The flow rate for the flooding was set at 0.2 mL min−1. The amount of displaced oil (mL) and displaced water (mL) in core flooding process were recorded.
Oil recovery efficiency (ORE) was calculated using the following equation: ORE (%) = total volume of oil displaced/volume of original oil in core × 100 (eqn (1)), where the volume of the original oil in place (mL) is the volume of brine displaced by oil saturation. Therefore, enhanced oil recovery efficiency (EORE) was calculated using the following equation: EORE (%) = ORE (%) at the end of the second water flooding − ORE (%) at the end of bacterial injection (eqn (2)).
Acknowledgements
This work was financially supported by the National High Technology Research and Development Program of China (no. 2013AA064402) and the Program of China Daqing Oilfield Company Limited.References
- M. P. Płociniczak, G. A. Płaza, G. Piotrowska-Seget and S. S. Cameotra, Int. J. Mol. Sci., 2011, 12, 633–654 CrossRef PubMed.
- R. Sen, Prog. Energy Combust. Sci., 2008, 34, 714–724 CrossRef CAS PubMed.
- L. R. Brown, Curr. Opin. Microbiol., 2010, 13, 316–320 CrossRef CAS PubMed.
- H. Amani, M. M. Müller, C. Syldatk and R. Hausmann, Appl. Biochem. Biotechnol., 2013, 170, 1080–1093 CrossRef CAS PubMed.
- J. F. B. Pereira, E. J. Gudiña, R. Costa, R. Vitorino, J. A. Teixeira, J. A. Coutinho and L. R. Rodrigues, Fuel, 2013, 111, 259–268 CrossRef CAS PubMed.
- M. J. McInerney, D. P. Nagle and R. M. Knapp, in Petroleum Microbiology, ed. B. Ollivier and M. Magot, ASM press, Washington, D. C, 2005, pp. 215–237 Search PubMed.
- N. Youssef, M. S. Elshahed and M. J. McInerney, Adv. Appl. Microbiol., 2009, 66, 141–251 CAS.
- M. M. Müller, J. H. Kügler, M. Henkel, M. Gerlitzki, B. Hörmann, M. Pöhnlein, C. Syldatk and R. Hausmann, J. Biotechnol., 2012, 162, 366–380 CrossRef PubMed.
- M. Gray, A. Yeung, J. Foght and H. W. Yarranton, Paper presented at the SPE Annual Technical Conference and Exhibition, Denver, 2008 Search PubMed.
- M. Nitschke, S. G. V. A. O. Cost and J. Contiero, Biotechnol. Prog., 2005, 21, 1593–1600 CrossRef CAS PubMed.
- M. Fulazzaky, D. I. Astuti and M. A. Fulazzaky, RSC Adv., 2015, 5, 3908–3916 RSC.
- I. Lazar, I. G. Petrisor and T. F. Yen, Pet. Sci. Technol., 2007, 25, 1353–1366 CrossRef CAS.
- N. Youssef, D. R. Simpson, K. E. Duncan, M. J. Mclnerney, M. Folmsbee, T. Fincher and R. M. Knapp, Appl. Environ. Microbiol., 2007, 73, 1239–1247 CrossRef CAS PubMed.
- N. Youssef, D. R. Simpson, M. J. Mclnerney and K. E. Duncan, Int. Biodeterior. Biodegrad., 2013, 81, 127–132 CrossRef CAS PubMed.
- M. J. McInerney, S. Maudgalya, D. P. Nagle and R. M. Knapp, Recent Res. Dev. Microbiol., 2002, 6, 269–284 CAS.
- M. Javaheri, G. E. Jenneman, M. J. McInerney and R. M. Knapp, Appl. Environ. Microbiol., 1985, 50, 698–700 CAS.
- J. D. Albino and I. M. Nambi, Adv. Mater. Res., 2010, 93, 623–626 CrossRef PubMed.
- F. Zhao, R. Shi, J. Zhao, G. Li, X. Bai, S. Han and Y. Zhang, J. Appl. Microbiol., 2015, 118, 379–389 CrossRef CAS PubMed.
- J. Y. Wu, K. L. Yeh, W. B. Lu, C. L. Lin and J. S. Chang, Bioresour. Technol., 2008, 99, 1157–1164 CrossRef CAS PubMed.
- G. Soberón-Chávez, F. Lepine and E. Déziel, Appl. Microbiol. Biotechnol., 2005, 68, 718–725 CrossRef PubMed.
- G. Hauser and M. L. Karnovsky, J. Biol. Chem., 1957, 224, 91–105 CAS.
- S. N. R. L. Silva, C. B. B. Farias, R. D. Rufino, J. M. Luna and L. A. Sarubbo, Colloids Surf., B, 2010, 79, 174–183 CrossRef CAS PubMed.
- P. Bharali, S. P. Singh, N. Dutta, S. Gogoi, L. C. Bora, P. Debnath and B. K. Konwar, RSC Adv., 2014, 4, 38698–38706 RSC.
- W. J. Xia, Z. B. Luo, H. P. Dong, L. Yu, Q. F. Cui and Y. Q. Bi, Appl. Biochem. Biotechnol., 2012, 166, 1148–1166 CrossRef CAS PubMed.
- F. Leitermann, C. Syldatk and R. Hausmann, J. Biol. Eng., 2008, 2, 13–20 CrossRef PubMed.
- H. Yin, J. Qiang, Y. Jia, J. S. Ye, H. Peng, H. M. Qin, N. Zhang and B. Y. He, Process Biochem., 2009, 44, 302–308 CrossRef CAS PubMed.
- A. Rabiei, M. Sharifinik, A. Niazi, A. Hashemi and S. Ayatollahi, Appl. Microbiol. Biotechnol., 2013, 97, 5979–5991 CrossRef CAS PubMed.
- Y. Kryachko, S. Nathoo, P. Lai, J. Voordouw, E. J. Prenner and G. Voordouw, Int. Biodeterior. Biodegrad., 2013, 81, 133–140 CrossRef CAS PubMed.
- Q. Wang, X. Fang, B. Bai, X. Liang, P. J. Shuler, W. A. Goddard III and Y. Tang, Biotechnol. Bioeng., 2007, 98, 842–853 CrossRef CAS PubMed.
- M. M. Müller and R. Hausmann, Appl. Microbiol. Biotechnol., 2011, 91, 251–264 CrossRef PubMed.
- F. Zhao, M. Mandlaa, J. Hao, X. Liang, R. Shi, S. Han and Y. Zhang, Lett. Appl. Microbiol., 2014, 59, 231–237 CrossRef CAS PubMed.
- J. Felsenstein, Evolution, 1985, 39, 783–789 CrossRef.
- D. G. Cooper and B. G. Goldenberg, Appl. Environ. Microbiol., 1987, 53, 224–229 CAS.
- M. T. Bao, X. P. Kong, G. C. Jiang, X. L. Wang and X. M. Li, J. Pet. Sci. Eng., 2009, 66, 42–46 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |