Template synthesis of carbon self-doped g-C3N4 with enhanced visible to near-infrared absorption and photocatalytic performance
Zaiwang Zhaoa,
Yanjuan Suna,
Fan Dong*a,
Yuxin Zhang*bc and
Han Zhaod
aChongqing Key Laboratory of Catalysis and Functional Organic Molecules, College of Environmental and Biological Engineering, Chongqing Technology and Business University, Chongqing, 400067, P. R. China. E-mail: dfctbu@126.com
bCollege of Materials Science and Engineering, Chongqing University, Chongqing 400044, P. R. China. E-mail: zhangyuxin@cqu.edu.cn
cNational Key Laboratory of Fundamental Science of Micro/Nano-Devices and System Technology, Chongqing University, Chongqing, 400044, P. R. China
dDepartment of Chemical and Biomolecular Engineering, Nanyang Technological University, Singapore 639793, Singapore
First published on 9th April 2015
Abstract
In order to fully address the low surface area, fast charge recombination and limited visible light absorption of pristine g-C3N4, we present a novel and straightforward strategy towards the synthesis of carbon self-doped g-C3N4 by using porous carbon foam as a soft-template. The C-doped g-C3N4 displayed a high BET surface area (65 m2 g−1), extended absorption ranging from visible light to near-infrared (800 nm) and accelerated electron–hole separation. The role of carbon doping on the band structure and electrical conductivity was revealed. The optimized C-doped g-C3N4 demonstrated an exceptionally high photocatalytic performance towards the purification of NO in air, and exceeded other reported visible-light photocatalysts, such as nonmetal-doped TiO2, BiOBr, (BiO)2CO3 and porous g-C3N4. This decent C-doped g-C3N4 photocatalyst also showed good photocatalytic stability for NO removal. The present work could provide new insights into the modification and understanding of self-doped semiconductor photocatalysts.
1 Introduction
The synthesis of efficient visible-light-driven (VLD) photocatalysts has provoked great attention in materials chemistry.1–15 Different types of photocatalysts are utilized in photocatalytic domains, including metal-containing photocatalysts (TiO2, AgPO4, Bi2WO6),3–6 metal-free photocatalysts (g-C3N4, S, P),7–18 SPR-mediated noble metal photocatalysts (Au, Ag)19–23 and non-noble metal photocatalysts (Bi, Cu).24–26 In particular, g-C3N4 is earth-abundant, robust and economically viable and has demonstrated great photocatalytic potential, thus attracting increasing interest.13–15 However, pristine g-C3N4 has a low surface area, fast charge recombination and can only utilize blue light up to 450 nm, which limits its large scale applications.Various strategies have been adopted to enhance the photocatalytic capability of g-C3N4.2,13,14,27 Among these strategies, doping is considered an effective method. For instance, boron-doping could improve the photocatalytic performance of g-C3N4 for methyl orange photodegradation owing to the increased light absorption of the catalyst and the promotion of electrical conductivity in g-C3N4.28 Sulfur-doping could enhance the photoreactivity of g-C3N4 owing to both the enlarged BET surface area and the promotion of light absorption.2 However, an anion-doping strategy is likely to have some detrimental shortcomings such as poor oxidation power of the photoexcited hole and fast recombination of the photogenerated electron–hole pairs due to the impurity arising from the defects induced by doping asymmetry.29
To date, it has been demonstrated that self-doping can not only fine-tune the surface architectures and electronic properties of semiconductor photocatalysts, but can also avoid the introduction of foreign impurities and defects which may act as recombination centers.30–34 For example, Jiang et al. successfully fabricated self-doped BiOCl with remarkable visible-light-driven photocatalytic activity.33 Yu and colleagues designed nitrogen self-doped nanosized TiO2 sheets with extremely high visible-light photocatalytic performance for hydrogen production.34 Moreover, the self-doping method has also been successfully reported for g-C3N4 systems and show excellent photocatalytic activities.27,35 Zhang et al. were the first to investigate carbon self-doped g-C3N4 theoretically and experimentally, and demonstrated that this carbon self-doped strategy can increase the visible-light absorption, electrical conductivity and surface area resulting in enhancing the photooxidation capability.27 These successes further demonstrated that carbon self-doping was indeed an effective and potential method to advance the photooxidative ability of g-C3N4. However, the photocatalytic mechanism of carbon self-doped g-C3N4 is still unclear and the application of carbon-doped g-C3N4 for air purification has not been reported so far.
Herein, we introduce an in situ one-pot strategy to prepare carbon self-doped g-C3N4 by using commercially available polyporous carbon foam as a template. The influence of the mass ratio of the precursors of melamine and the carbon foam was also investigated. To evaluate the photocatalytic performance, all of the as-prepared carbon self-doped g-C3N4 samples were applied to the photocatalytic removal of NO from air using visible light irradiation. The optimized sample demonstrated excellent visible-light photocatalytic capability, which was ascribed to the enlarged BET surface area, the hindering of recombination of photoexcited carriers, and the enhanced light absorption ranging from visible light to near-infrared.
2 Experimental section
2.1 Catalyst preparation
All the reagents employed in this study were of analytical grade and used without further purification. In a typical synthesis, melamine powders of different masses (2.0, 5.0 and 8.0 g) were put into three different alumina crucibles (50 mL). Then 20 mL deionized water was added into the alumina crucibles, and heated and stirred until complete dissolution. Afterwards, 0.3 g melamine porous resin foam (MRF, supplied by Puyang Green Universe Chemical Co., Ltd.) was cut into small pieces and added to each of the solutions. After recrystallization at 100 °C, the alumina crucibles were placed in a muffle furnace, and heated at 550 °C for 2 h with a heating rate of 15 °C min−1. After the reaction, the alumina crucibles were cooled to room temperature. The resultant g-C3N4 samples were collected and ground into powders. The different g-C3N4 samples prepared from different precursor masses (2, 5 and 8 g) with MRF were labeled as 2M-CF, 5M-CF and 8M-CF, respectively. Pure g-C3N4 as a reference was also prepared by direct pyrolysis of 5 g melamine without adding MRF, and labeled as 5M.2.2 Characterization
The X-ray diffraction (XRD) patterns of the samples were obtained using an X-ray diffractometer equipped with intense Cu Kα radiation (Model D/max RA, Rigaku Co., Japan). The carbon to nitrogen ratios (C/N) of the photocatalysts were detected by elemental analyses (EA, Vario ELIII CHNSO). The morphology, structure and chemical composition of the obtained products were analyzed using scanning electron microscopy (SEM, JEOL model JSM-6490, Japan) and transmission electron microscopy (TEM, JEM-2010, Japan). The Brunauer–Emmett–Teller (BET) specific surface areas (SBET) of the samples were determined using nitrogen adsorption apparatus (ASAP 2020, USA) with all samples degassed at 100 °C for 12 h prior to measurements. X-ray photoelectron spectroscopy (XPS) measurements were carried out to investigate the surface chemical compositions and states with an Al Kα X-ray (hν = 1486.6 eV) radiation source operated at 150 W (Thermo ESCALAB 250, USA). The UV-vis diffuse reflection spectra (UV-vis DRS) were obtained using dry-pressed disk samples in a Scan UV-vis spectrophotometer (UV-2450, Shimadzu, Japan) with 100% BaSO4 as the standard sample. Photoluminescence (PL) studies (F-7000, HITACHI, Japan) were conducted to investigate the optical properties of the obtained samples.2.3 Evaluation of photocatalytic performance
The photocatalytic capabilities of the as-synthesized samples were evaluated by removal of NO at ppb level in a continuous flow reactor (Scheme 1). The reactor was 4.5 L (30 cm × 15 cm × 10 cm), made of polymeric glass, and covered with Saint-Glass. A commercial tungsten halogen lamp (100 W) was placed 20 cm vertically above the reactor. A UV cut-off filter (420 nm) was applied to remove UV light from the tests of visible light photocatalytic activity. The as-prepared sample (0.20 g) was dispersed in distilled water (50 mL) by 10 min of ultrasonic treatment and then coated onto two glass dishes (12.0 cm in diameter). The coated dishes were then pretreated at 70 °C to remove water from the suspension and, after cooling down to room temperature, were placed at the centre of the reactor. NO gas was supplied from a compressed gas cylinder at a concentration of 100 ppm of NO (N2 balance). The initial concentration of NO was diluted to about 600 ppb via air streaming. The flow rates of the air stream and NO were controlled at 2.4 L min−1 and 15 mL min−1, respectively and the two gas streams were then premixed in a three-way valve. The relative humidity of the air stream was controlled at 50%. When the adsorption–desorption equilibrium had been achieved, the lamp was turned on. The concentration of NO was measured every one min using a NOx analyzer (Thermo Scientific, 42i-TL), which also monitored the concentration of NO2 and NOx (NOx represents NO + NO2). The removal ratio (η) of NO was calculated using η (%) = (1 − C/C0) × 100%, where C is the outlet concentration of NO after reaction for time t, and C0 represents the inlet concentration after reaching the adsorption–desorption equilibrium. The photocatalytic NO removal reaction has pseudo-first-order reaction kinetics at low NO concentrations as ln(C0/C) = kappt, where kapp is the apparent rate constant.3 Results and discussion
3.1 Phase structures and chemical constituents
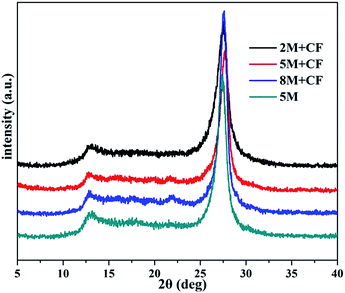
Two typical diffraction peaks ascribed to g-C3N4 (JCPDS 87-1526) were detected (Fig. 1), both in the g-C3N4 and the carbon self-doped g-C3N4 samples, demonstrating that carbon self-doping does not change the architecture of g-C3N4. The stronger peak centered at 27.1° was ascribed to the (002) diffraction peak reflecting the interplanar graphitic stacking and the minor peak at around 13.1° can be indexed as the (100) diffraction peak relating to the in-plane tri-s-triazine units.15 Elemental analyses were also performed to test the molar ratios of carbon to nitrogen (C/N) in both the pure and C-doped g-C3N4. The C/N value of g-C3N4 is 0.561, while a slight enhancement in the C/N value to 0.563, 0.571 and 0.590 for C-doped 8M-CF, 5M-CF and 2M-CF, respectively can be observed. This gradual increase in C/N ratio revealed that carbon has been successfully incorporated into g-C3N4. This phenomenon of progressively increasing carbon content in g-C3N4 was also reported by other groups27 (the C/N value of 0.751 for g-C3N4 and 0.766 for C-doped g-C3N4).FT-IR spectra were utilized to confirm the molecular structure of all the samples. As shown in Fig. 2, the strong absorption peak around 700–800 cm−1 can be observed and is assigned to the bending vibration mode of CN heterocycles, while the peak centered at 810 cm−1 is the characteristic plane bending vibration mode of the triazine units (Fig. 2a).36 The peaks observed at 1200–1600 cm−1 are mainly due to stretching modes of C–N heterocycles, and the broad bands between 3000–3700 cm−1 (Fig. 2b) are assigned to adsorbed H2O molecules and N–H vibrations.37
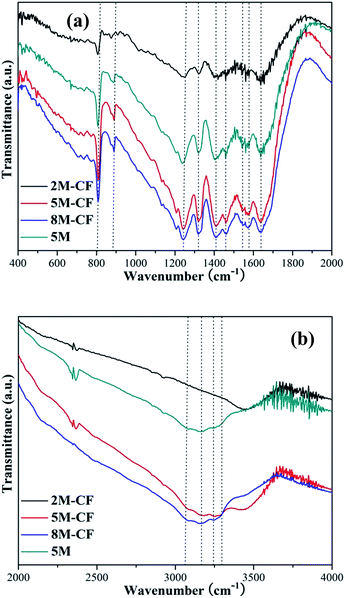 | ||
| Fig. 2 FT-IR spectra of the pure and C-doped g-C3N4 samples in the range of (a) 400–2000 cm−1 and (b) 2000–4000 cm−1. | ||
XPS was also carried out to determine the chemical compositions of pure g-C3N4 and C-doped g-C3N4. As shown in Fig. 3a, the XPS spectra of C 1s of the 5M and 5M-CF samples exhibit two typical peaks centered at binding energies of 284.7 and 288.2 eV, which are characteristic of C–N–C coordination and C–C bonds, respectively.29,38 The area ratios of the two typical peaks at 284.7 and 288.2 eV were calculated to be 0.095 and 0.132 for 5M and 5M-CF, respectively. Three peaks can be observed in the N 1s spectra of the two samples (Fig. 3b). The major peaks centered at 400.6 and 398.4 eV can be indexed to the N–C3 and C–N–C groups, respectively29 and the peak area ratios are 0.183 for g-C3N4 and 0.170 for carbon self-doped g-C3N4. These results, in addition to the shift of the main C and N peaks, reflect that the bridging N have been replaced by C due to the self-doping by carbon (Fig. 3c and d).29 Zhang’s group reported that large, delocalized π bonds will be formed between the substituted carbons and the hexatomic rings when the bridging N atoms are replaced by C atoms,29 which would enhance the electrical conductivity of g-C3N4. These delocalized π bonds are favorable for electron movement and transfer, resulting in the impeding of photoexcited electron–hole recombination.29,38 The present result is consistent with previous reports.29,38 The concentration of the C dopant was estimated to be 7% on the basis of the XPS result.
3.2 Morphology and mesoporous structure
The carbon self-doping strategy can not only tune the electron architecture, but can also exert a significant influence on the microstructure. Fig. 4 shows the morphology of the as-synthesized g-C3N4 and the carbon-doped g-C3N4 samples. As can be seen, the pure 5M sample shows an aggregated morphology with irregular layers (Fig. 4a and b). After introducing the carbon foam as a soft template, the sample is transformed into many ever smaller, near-spherical nanoparticles as the mass ratio of the carbon foams and g-C3N4 is increased. The decreased size of the g-C3N4 layers is ascribed to the severance of the large layers, which is caused by the bridging N atoms being replaced, as shown by the XPS results. The TEM and high magnification TEM results (Fig. 5) also demonstrate the irregular morphology of g-C3N4 layers. The porous architecture is confirmed by N2 adsorption–desorption isotherms and BJH characterizations (Fig. 6a and b). The BET surface areas are measured to be 11, 17, 65 and 14 m2 g−1 for 5M, 2M-CF, 5M-CF and 8M-CF, respectively (Table 1). A mesoporous architecture with a pore size distribution centered at 3.5 nm can be observed for all samples, while notably, a wide pore size distribution ranging from 3 to 50 nm for 5M-CF can be found. The enhanced BET surface area provides more active sites for absorptions and the mesoporous structures are beneficial for the transfer of reactants, resulting in high photocatalytic performances. | ||
| Fig. 4 SEM and magnified view of 5M (a and b), 2M-CF (c and d), 5M-CF (e and f) and 8M-CF (g and h). | ||
 | ||
| Fig. 6 (a) N2 adsorption–desorption isotherms of 5M, 2M-CF, 5M-CF, 8M-CF and (b) the corresponding pore-size distribution curves. | ||
| Sample name | SBET (m2 g−1) | Pore volume (cm3 g−1) | Peak diameter (nm) | Band gap (eV) | NO η (%) |
|---|---|---|---|---|---|
| 5M | 11 | 0.080 | 22.4/30.3 | 2.62 | 14.3 |
| 2M-CF | 17 | 0.082 | 12.7/19.1 | 1.87 | 8.1 |
| 5M-CF | 65 | 0.293 | 13.9/18.0 | 2.05 | 50.1 |
| 8M-CF | 14 | 0.085 | 14.4/24.2 | 2.24 | 22.3 |
3.3 Optical absorption and band structure
DRS UV-vis absorption spectra (Fig. 7) of the as-synthesized photocatalysts in the wavelength range of 300–800 nm were recorded. As can be seen in Fig. 7a, the absorption edge of pure g-C3N4 is at 470 nm, which agrees with previous literature.15 After carbon doping, the absorption is largely enhanced in the UV light to near-infrared light range. Furthermore, the light absorption intensity increased with increasing carbon doping. The light absorption response is consistent with the color variations of the samples (inset, Fig. 7a). The band gaps are 2.62, 2.24, 2.05 and 1.87 eV for 5M, 8M-CF, 5M-CF and 2M-CF respectively, which are estimated by the plots of (αhν)1/2 versus photon energy of the samples (Fig. 7b). The diminished band gaps can be ascribed to the negative shift of the valence band position (Fig. 7c) relative to that of pristine g-C3N4, and are detected by valence band XPS (+1.57 eV for 5M, +1.23 eV for 5M-CF). The decreased band gaps induced by carbon self-doping effectively enlarge the light absorption ranges and strengthen the light absorption abilities, which is expected to enhance the photocatalytic performances. | ||
| Fig. 7 (a) UV-vis DRS and (b) (αhν)1/2 versus band gap plots of 5M, 2M-CF, 5M-CF, 8M-CF; (c) the valence band XPS of the 5M and 5M-CF samples. | ||
3.4 Charge separation
Generally, there are two fates for the photo-driven electron–hole pairs after photoexcitation. The first is to transfer to the active sites on the surface of the photocatalyst for subsequent chemical reaction. The second is to recombine with each other without further reaction. Photoluminescence (PL) spectra of the samples were used to investigate the fate of photoexcited electrons and holes using an excitation light source of 330 nm at room temperature (Fig. 8). The results show that pure g-C3N4 has a high electron–hole recombination intensity, while the recombination rate is severely hampered after carbon-doping. The clear suppression in the recombination of photo-induced electron–hole pairs is mainly due to the increased electrical conductivity of g-C3N4 induced by carbon self-doping arising from the formation of large, delocalized π bonds.27 Furthermore, Zhang’s group also demonstrated theoretically and experimentally that carbon self-doping could enhance the electrical conductivity of g-C3N4 due to the construction of large, delocalized π bonds.27 In general, low photo-driven electron–hole recombination could result in a high photocatalytic capability owing to the generation of more active species which is a crucial factor for subsequent photocatalytic reaction.3.5 Electrical conductivity
The enhanced electrical conductivity of C-doped g-C3N4 was demonstrated by electrochemical impedance spectroscopy (EIS) measurements (Fig. 9). This reduced arc radius shows that carbon self-doping can significantly enhance the electrical conductivity of g-C3N4 due to the formation of large, delocalized π bonds formed by the homogeneous substitution of bridging N atoms by C atoms.27 | ||
| Fig. 9 Nyquist plots for the 5M-CF and 5M samples under visible light irradiation (λ > 420 nm, [Na2SO4] = 0.5 M). | ||
3.6 Photocatalytic properties
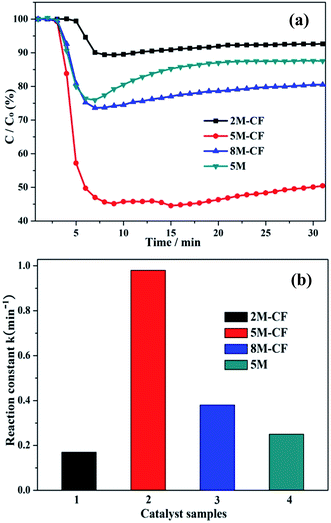
We first investigated the photocatalytic activity (Fig. 10) of the as-prepared samples by gas phase NO purification (at a ppb level) in a continuous reactor under visible-light illumination. Previous results have shown that NO is stable and cannot be photooxidized under light illumination without a photocatalyst, or in the presence of a photocatalytic material without any light irradiation.15 NO can be transformed to HNO3 as the final product because the photooxidation is driven by the photogenerated reactive radicals under light irradiation.14 For pure g-C3N4 (Fig. 10a), the photocatalytic performance is measured by the removal ratio of NO and is about 14.3% and reaches an equilibrium after 30 min irradiation. The photocatalytic activities of C-doped g-C3N4 are 22.3, 50.1 and 8.1% for 8M-CF, 5M-CF and 2M-CF, respectively. Notably, the photodegradation ability of the optimized carbon-doped 5M-CF has increased to 50.1%, and outperforms those of BiOBr (21.3%),39 C-doped TiO2 (21.8%),40 N-TiO2 (36.5%),40 (BiO)2CO3 (43.5%)41 and porous g-C3N4 (32.7%).42 To further comprehend the reaction kinetics of the NO degradation catalyzed by the pure and C-doped g-C3N4 samples, the experimental data were fitted with a first-order model (Fig. 10b).43 The photodegradation constant was calculated to be 0.95 min−1 for 5M-CF, which is 3.8 times than that of 5M. The increased photocatalytic performance of 5M-CF is due to the contributions from the following factors: (1) the improved SBET surface area provides more active sites for NO absorption, (2) the enhanced light absorption ability in the visible to near-infrared range which can induce more active species for photooxidative reactions; (3) lastly, but significantly, the enhanced electrical conductivity of g-C3N4 due to the large, delocalized π bonds resulting in fewer photogenerated electron–hole recombination. These findings indicate that carbon self-doping is an effective strategy for advancing g-C3N4 materials with high photocatalytic performances. The decrease in the photodegradation ability of 8M-CF (compared to 5M-CF) can be ascribed to the decreased SBET surface reducing the number of active sites and to the near-spherical particles, which increase the distance for photoexcited electrons and holes to move to the surface of the photocatalyst, compared with that of nanosheet structures.In addition, the stability of 5M-CF was further investigated by carrying out the photocatalytic reaction over multiple runs and is shown in Fig. 11. The photocatalytic performance slightly declines after four repeated runs, which is ascribed to the enrichment of reaction products on the surface of the photocatalyst. However, no obvious deactivation can be observed, demonstrating the promising photocatalytic stability of this catalyst for NO removal.44–47
4 Conclusion
In summary, (1) we developed an in situ one-pot strategy to obtain carbon-rich g-C3N4 by using a commercially available carbon foam as a soft-template. The morphological architectures, and the intrinsic electronic and band structures of g-C3N4 can be fine-tuned by substitution of lattice nitrogen with carbon.(2) The extent of carbon self-doping can significantly enhance the photocatalytic performance for the removal of NO in air under visible light.
(3) The clearly improved photocatalytic capability is assigned to the contributions from the improved SBET surface area, the accelerated electrical conductivity, and the retarded photogenerated electron–hole recombination arising from the formation of large, delocalized π bonds. The optimised C-doped g-C3N4 photocatalyst also showed good photocatalytic stability. This work could provide new perspectives for the design and synthesis of self-doped photocatalysts with enhanced performances.
Acknowledgements
This research is financially supported by the National Natural Science Foundation of China (51478070, 51108487), and the Science and Technology Project from Chongqing Education Commission (KJ1400617).Notes and references
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76 CrossRef CAS PubMed.
- G. Liu, P. Niu, C. H. Sun, S. C. Smith, Z. G. Chen, G. Q. Lu and H. M. Cheng, J. Am. Chem. Soc., 2010, 132, 11642 CrossRef CAS PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS PubMed.
- Y. P. Bi, S. X. Ouyang, N. T. Umezawa, J. Y. Cao and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS PubMed.
- L. W. Zhang and Y. F. Zhu, Catal. Sci. Technol., 2012, 2, 694 CAS.
- S. M. Sun and W. Z. Wang, RSC Adv., 2014, 4, 47136 RSC.
- G. P. Dong, Y. H. Zhang, Q. W. Pan and J. R. Qiu, J. Photochem. Photobiol., C, 2014, 20, 33 CrossRef CAS PubMed.
- J. J. Zhu, P. Xiao, H. L. Li and S. A. C. Carabineiro, ACS Appl. Mater. Interfaces, 2014, 6, 16449 CAS.
- J. S. Zhang, B. Wang and X. C. Wang, Progress in Chemistry, 2014, 26, 19 CAS.
- Y. L. Tian, B. B. Chang, Z. C. Yang, B. C. Zhou, F. N. Xi and X. P. Dong, RSC Adv., 2014, 4, 4187 RSC.
- Y. Y. Bu and Z. Y. Chen, RSC Adv., 2014, 4, 45397 RSC.
- Y. G. Li, X. L. Wei, H. J. Li, R. R. Wang, J. Feng, H. Yun and A. N. Zhou, RSC Adv., 2014, 1 Search PubMed.
- X. H. Li, X. C. Wang and M. Antonietti, ACS Catal., 2012, 2, 2082 CrossRef CAS.
- Z. W. Zhao, Y. J. Sun and F. Dong, Nanoscale, 2015, 7, 15 RSC.
- F. Dong, Z. W. Zhao, T. Xiong, Z. L. Ni, W. D. Zhang, Y. J. Sun and W. K. Ho, ACS Appl. Mater. Interfaces., 2013, 5, 11392 CAS.
- G. Liu, P. Niu, L. C. Yin and H. M. Cheng, J. Am. Chem. Soc., 2012, 134, 9070 CrossRef CAS PubMed.
- Z. R. Shen, Z. F. Hu, W. J. Wang, S. F. Lee, D. K. L. Chan, Y. C. Li, T. Gu and J. C. Yu, Nanoscale, 2014, 6, 14163 RSC.
- N. Tian, H. Huang, Y. He, Y. Guo and Y. Zhang, RSC Adv., 2014, 4, 42716 RSC.
- Y. A. Attia, D. Buceta, C. Blanco-Varela, M. B. Mohamed, G. Barone and M. A. Ló pez-Quintela, J. Am. Chem. Soc., 2014, 136, 1182 CrossRef CAS PubMed.
- Y. Di, X. C. Wang, A. Thomas and M. Antonietti, ChemCatChem, 2010, 2, 834 CrossRef CAS PubMed.
- X. H. Li, X. C. Wang and M. Antonietti, Chem. Sci., 2012, 3, 2170 RSC.
- Y. F. Zhang, F. Mao, H. J. Yan, K. W. Liu, H. M. Cao, J. G. Wu and D. Q. Xiao, J. Mater. Chem. A, 2015, 3, 109 CAS.
- L. Ge, C. C. Han, J. Liu and Y. F. Li, Appl. Catal., A, 2011, 409–410, 215 CrossRef CAS PubMed.
- F. Dong, T. Xiong, Y. J. Sun, Z. W. Zhao, Y. Zhou, X. Feng and Z. B. Wu, Chem. Commun., 2014, 50, 10386 RSC.
- F. Dong, Q. Y. Li, Y. J. Sun and W. K. Ho, ACS Catal., 2014, 4, 4341 CrossRef CAS.
- S. Paria and O. Reiser, ChemCatChem., 2014, 6, 2477 CrossRef CAS PubMed.
- G. H. Dong and L. Z. Zhang, J. Mater. Chem., 2012, 22, 1160 RSC.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894 CrossRef CAS PubMed.
- G. H. Dong, K. Zhao and L. Z. Zhang, Chem. Commun., 2012, 48, 6178 RSC.
- F. Zuo, L. Wang, T. Wu, Z. Y. Zhang, D. Borchardt and P. Y. Feng, J. Am. Chem. Soc., 2010, 132, 11856 CrossRef CAS PubMed.
- K. Xie, N. T. Umezawa, N. Zhang, P. P. Reunchan, Y. J. Zhang and J. H. Ye, Energy Environ. Sci., 2011, 4, 4211 CAS.
- J. Q. Wang, S. Y. Su, B. Liu, M. H. Cao and C. W. Hu, Chem. Commun., 2013, 49, 7830 RSC.
- J. Jiang, L. Z. Zhang, H. Li, W. W. He and J. J. Yin, Nanoscale, 2013, 5, 10573 RSC.
- Q. J. Xiang, J. G. Yu, W. G. Wang and M. Jaroniec, Chem. Commun., 2011, 47, 6906 RSC.
- P. Zhang, X. H. Li, C. L. Shao and Y. C. Liu, RSC Adv., 2015, 5, 15621 RSC.
- F. Dong, Z. Y. Wang, Y. J. Sun, W. K. Ho and H. D. Zhang, J. Colloid Interface Sci., 2013, 401, 70 CrossRef CAS PubMed.
- J. H. Liu, T. K. Zhang, Z. C. Wang, G. H. Dawson and W. Chen, J. Mater. Chem, 2011, 21, 14398 RSC.
- A. Iraqi, J. A. Crayston and J. C. Walton, J. Mater. Chem., 1998, 8, 31 RSC.
- W. D. Zhang, Q. Zhang and F. Dong, Ind. Eng. Chem. Res., 2013, 52, 6740 CrossRef CAS.
- F. Dong, Y. H. Li, W. K. Ho, H. D. Zhang, M. Fu and Z. B. Wu, Chin. Sci. Bull., 2014, 59, 688 CrossRef CAS PubMed.
- F. Dong, S. C. Lee, Z. B. Wu, Y. Huang, M. Fu, W. K. Ho, S. C. Zou and B. Wang, J. Hazard. Mater, 2011, 195, 346 CrossRef CAS PubMed.
- F. Dong, M. Y. Ou, Y. K. Jiang, S. Guo and Z. B. Wu, Ind. Eng. Chem. Res., 2014, 53, 2318 CrossRef CAS.
- S. Zheng, W. J. Jiang, M. Rashid, Y. Cai, D. D. Dionysiou and K. E. O’Shea, Molecules, 2015, 20, 2622 CrossRef CAS PubMed.
- F. Dong, Z. Wang, Y. Li, W. Ho and S. Lee, Environ. Sci. Technol., 2014, 48, 10345 CrossRef CAS PubMed.
- T. Xiong, H. Huang, Y. Sun and F. Dong, J. Mater. Chem. A, 2015, 3, 6118 CAS.
- Z. Wang, W. Guan, Y. Sun, F. Dong, Y. Zhou and W. K. Ho, Nanoscale, 2015, 7, 2471 RSC.
- F. Dong, H. T. Liu, W. K. Ho, M. Fu and Z. B. Wu, Chem. Eng. J., 2013, 214, 198 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |