Adsorption of chlorinated phenols on multiwalled carbon nanotubes
Abstract
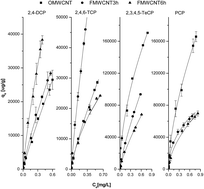
This work studies the adsorption of four chlorinated phenols (2,4-dichlorophenol, 2,4,6-trichlorophenol, 2,3,4,5-tetrachlorophenol and pentachlorophenol) in aqueous solutions on multiwalled carbon nanotubes (MWCNT). To investigate the influence of oxygen containing functional groups, adsorption parameters for the phenols were determined for original MWCNT (OMWCNT) and functionally modified MWCNT (FMWCNT) by acid treatment for 3 h and 6 h. The correlation between phenol adsorption affinity and specific surface area (SSA) indicates that OMWCNT have higher adsorption affinities for larger molecules such as tetrachlorophenol and pentachlorophenol, which suggests that mesopore filling is not the dominant mechanism controlling their adsorption. Electrostatic repulsion between disassociated chlorinated phenols and disassociated functional groups on the surface of both FMWCNT lead to adsorption decreasing with increasing functionalisation under neutral pH conditions. On OMWCNT, a positive correlation between molecular hydrophobicity and adsorption affinity was obtained, indicating hydrophobic interactions control the adsorption of chlorinated phenols. To investigate the role of π–π interactions, Kd values (at 0.01 and 0.5 SW) were normalized by hydrophobicity. The Kd/KOW values for all MWCNT decreased from 2,4-dichlorophenol to pentachlorophenol and were negatively correlated with the electron-acceptor property of the molecules. The most pronounced π–π interactions were observed for 2,6-dichlorophenol on all MWCNT.

 Please wait while we load your content...
Please wait while we load your content...