Efficient aqueous dye-sensitized solar cell electrolytes based on a TEMPO/TEMPO+ redox couple†
Abstract
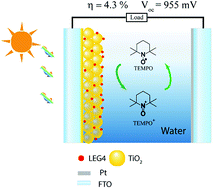
Aqueous electrolyte-based dye-sensitized solar cells (DSSCs) have recently emerged and shown to be a promising eco-friendly photovoltaic device. In the present study, we, for the first time, have developed 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and TEMPO+ tetrafluoroborate salt as a redox couple in an aqueous electrolyte for DSSCs. With the hydrophobic dye LEG4 as a light absorber, we have achieved a power conversion efficiency of 4.3% and a record open circuit voltage of 955 mV in the device. This is attributed to the high formal redox potential of TEMPO/TEMPO+ (0.71 V vs. NHE) in water. In addition, despite the wide use of surfactants in previous studies, we have clearly shown that the addition of surfactants to the electrolyte is detrimental to solar cell performance. Therefore, the use of surfactants in aqueous DSSC electrolytes should be avoided or used with caution.

 Please wait while we load your content...
Please wait while we load your content...