Response of extracellular polymeric substances to the toxicity of 2,4-dichlorophenol in aerobic granular sludge system: production and interaction mechanism
Yifan Wanga,
Dong Wei*ac,
Kai Lia,
Bingfeng Wanga,
Li Shiab,
Ge Zhanga,
Xiaodong Wangac,
Bin Du*ab and
Qin Weib
aSchool of Resources and Environment, University of Jinan, Jinan 250022, PR China. E-mail: dubin61@gmail.com; weidong506@163.com; Fax: +86 531 8276 7370; Tel: +86 531 8276 7370
bKey Laboratory of Chemical Sensing & Analysis in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, PR China
cShan Dong Lan Xi Environmental Protection Technology Co., Ltd, Jinan 250022, PR China
First published on 1st April 2015
Abstract
The objective of this study was to investigate the response of extracellular polymeric substances (EPS) to the toxicity of 2,4-dichlorophenol (2,4-DCP) in an aerobic granular sludge system. Results implied that the presence of 2,4-DCP could cause toxicity in the performance of biological nitrogen removal. Compared with the control experiment, NH4+–N removal efficiency decreased to 37.27 and 20.71% after the exposure of 2,4-DCP of 20 and 50 mg L−1, respectively. The main components of EPS, including polysaccharides and proteins, generally increased from 35.22 ± 0.69 to 38.25 ± 1.46 mg g−1 SS and 76.28 ± 0.34 to 83.30 ± 0.31 mg g−1 SS in the presence of 2,4-DCP. Three-dimensional excitation–emission matrix (3D-EEM) fluorescence spectroscopy as well as synchronous fluorescence spectra was used to evaluate the interaction mechanism between EPS and 2,4-DCP. 3D-EEM showed that the intensities of EPS obviously decreased with the increase of 2,4-DCP concentration. According to synchronous fluorescence spectra, the mechanism of fluorescence quenching involves to static quenching with a formation constant (KA) of 3.46 × 103 L mol−1. Fourier transform infrared spectroscopy (FTIR) was used to evaluate the change of functional groups of EPS samples before and after the addition of 2,4-DCP.
1. Introduction
Aerobic granular sludge is regarded as one of novel environmental biotechnological processes in the field of wastewater treatment due to its reliable and efficient capacity to produce high-quality effluent. Compared with the conventional activated sludge, aerobic granular sludge has a higher biomass concentration, a denser and stronger microbial aggregate structure, and more excellent settling capacity.1 Therefore, aerobic granular sludge-based technology has been successfully applied to the treatment of various wastewaters, including high strength wastewater containing organics, nitrogen, phosphorus and heavy metals.2,3Extracellular polymeric substances (EPS) are one of complex high-molecular-weight mixture of polymers, which are considered as the major component in all types of bioflocs and biofilm. EPS are mainly comprised by proteins (PN), polysaccharides (PS), nucleic acids, lipids, and phospholipids etc.4 It is well known that EPS play an important role in the formation and stable operation of aerobic granulation process. Zhu et al.5 found that aromatic protein-like substances are important in matured granular sludge, especially tyrosine in maintaining the stable structure of the granular sludge. Wei et al.6 cultivated aerobic granular sludge in a sequencing batch reactor (SBR) for simultaneously treating wastewater containing nitrogen and phosphorus, implying that PN and PS concentrations of EPS increased from 60.2 and 12.5 mg L−1 to 101.1 and 15.8 mg L−1, respectively.
Moreover, EPS have a strong binding capacity together with toxic substances, which is considered to be an important strategy to protect microorganism against harsh environment.7 Toxic substances mainly exist in industrial and municipal wastewater causing a negative impact on biological nitrogen removal.8 Comte et al.9 assessed the biosorption properties of EPS extracted from two different activated sludges towards Cd, Cu and Pb by using differential pulse polarography, suggesting that the number of EPS binding sites increased with the increase of the pH values. Therefore, it is important to investigate the interaction between EPS and toxic substances in aerobic granular sludge system during the wastewater treatment.
In this study, 2,4-dichlorophenol (2,4-DCP) was selected as a target pollutant to evaluate the potential toxicity to the performance of aerobic granular sludge system. As one of chlorinated phenolic compounds, 2,4-DCP is widely used in the synthesis of various more highly chlorinated phenols and pesticides. The potential toxicity of 2,4-DCP is of concern as its direct or indirect effects on aquatic ecosystems and animal populations.10 To achieve this purpose, fluorescence spectroscopy, including three-dimensional excitation–emission matrix (3D-EEM) and synchronous fluorescence, was applied to explore the interaction and mechanism between EPS and 2,4-DCP. The obtained result could provide useful information for understanding the response of aerobic granular sludge as well as the variety of EPS in the presence of toxic compounds.
2. Materials and methods
2.1 Reactor and synthetic wastewater
Aerobic granular sludge was obtained from a lab-scale SBR (17 L) operated over 1 year. The SBR was operated in successive cycle of 6 h each, consisting of 5 min for influent, 325 min for aeration, 10 min for settling and 20 min for effluent and idle. The compositions of synthetic wastewater were listed as follows: COD (chemical oxygen demand, as C6H12O6) 600 mg L−1; NH4+–N (as NH4Cl) 200 mg L−1, P (as K2HPO4) 15 mg L−1; CaCl2, 40 mg L−1; MgSO4·2H2O, 20 mg L−1; FeSO4·H2O, 20 mg L−1 and trace element solution 1.0 mg L−1 according to the previous literature.11 The pH value of influent wastewater was adjusted to about 7.5 by using NaHCO3 and HCl.2.2 Experimental design
Four batches of 500 mL beakers were used to conduct the toxicity assessment experiment. Firstly, 50 mL aerobic granular sludge was carried out from SBR at the end of the aeration phase, and resuspended into 250 mL by using deionized water. Next, 250 mL synthetic wastewater and different volumes of pre-determined 2,4-DCP solution (500 mg L−1) were successively added into each beater. After that, the final COD and NH4+–N concentrations were about 300 and 100 mg L−1 of each beaker, respectively. Four test concentrations of 2,4-DCP (0, 5, 20, 50 mg L−1) were used according to the literature reported by Zheng et al.12 The mixed liquor suspended solids (MLSS) concentration and sludge volume index (SVI) of each beaker was about 5.0 g L−1 and 20 mL g−1, respectively. The air was supplied by using an aeration pump with a constant airflow of 0.3 L min−1, resulting in the dissolved oxygen (DO) in each beaker was above 2 mg L−1.2.3 EPS extraction and 3D-EEM
EPS samples were extracted by using a modified heating extraction method as described previously.13 Briefly, 0.5 g aerobic granular sludge was first washed three times and resuspended into 30 mL deionised water, and then centrifuged in a 50 mL tube at 8000 rpm for 5 min to remove supernatant. Next, the cell pellet was re-suspended with 0.05% NaCl solution and heated at 80 °C for 30 min and then centrifuged at 8000 rpm for 15 min. The supernatant was collected and regarded as the EPS of aerobic granules.The polysaccharides (PS) content was measured by using anthrone–sulfuric acid method with glucose as the standard, while the protein (PN) content was measured by using the modified Lowry method with bovine serum albumin (BSA) as the standard.14 3D-EEM fluorescence spectra of EPS samples were obtained by using a luminescence spectrometry (LS-55, Perkin-Elmer Co., USA). EEM spectra were collected with subsequent scanning emission spectra from 280 to 550 nm at 0.5 nm increment by changing the excitation wavelength from 200 to 400 nm at 10 nm increment.
2.4 Binding test between 2,4-DCP and EPS
Firstly, 5 mL extracted EPS were added into 10 mL cube. Next, different volume of pre-determined 2,4-DCP (500 mg L−1) were added and mixed into a 10 mL tube to ensure 2,4-DCP concentration at 0–100 mg L−1. Finally, the mixed solution was set for 1 h for equilibrium before spectral analysis. Synchronous fluorescence spectra of EPS samples were collected by simultaneously scanning the excitation and emission wavelength from 200 to 450 nm with a constant offset (Δλ at 60 nm).2.5 Analytical methods
MLSS, NH4+–N, NO2−–N, NO3−–N concentrations were determined according to the standard methods.15 Fourier transform infrared spectroscopy (FTIR) was analyzed by using a FTS-165 spectrometer (Perkin-Elmer, USA). Before FTIR analysis, EPS samples were freeze-dried at −60 °C. FTIR spectra were measured on KBr pellets prepared by pressing mixtures of 1 mg dry powdered sample and 100 mg spectrometry grade KBr in a vacuum with precautions taken to avoid moisture uptake.All the samples were monitored immediately in this study. The experiments were analyzed in triplicate, and the averaged data were presented here. Statistical analyses were performed by using SPSS version 19.0 software. An analysis of variance (ANOVA) was used to test the significance of results and p < 0.05 was considered to be statistically significant, as similarly reported by Zheng et al.12
3. Results and discussion
3.1 Toxicity of 2,4-DCP on biological nitrogen removal
In order to understand the toxicity of 2,4-DCP on the performance of biological nitrogen removal in aerobic granular sludge system, the variations of NH4+–N, NO2−–N and NO3−–N concentrations were tested in cycles in the presence of 2,4-DCP at 0, 5, 20, 50 mg L−1. As shown in the control experiment (Fig. 1A), the NH4+–N concentration gradually decreased from 91.35 to 2.43 mg L−1 under the supply of aeration, resulting in the NH4+–N removal efficiency high of 97.33% at 300 min. To be more detailed, the maximum nitrite peak (29.00 mg L−1) was observed in 180 min and gradually deceased to 3.24 mg L−1 at the end of aeration process. Meanwhile, nitrate concentration successively increased to 82.53 mg L−1 with the decrease of nitrite. The results suggested that aerobic granular sludge system had a high nitrification activity for treating nitrogen-rich wastewater, as similarly reported by Peng et al.16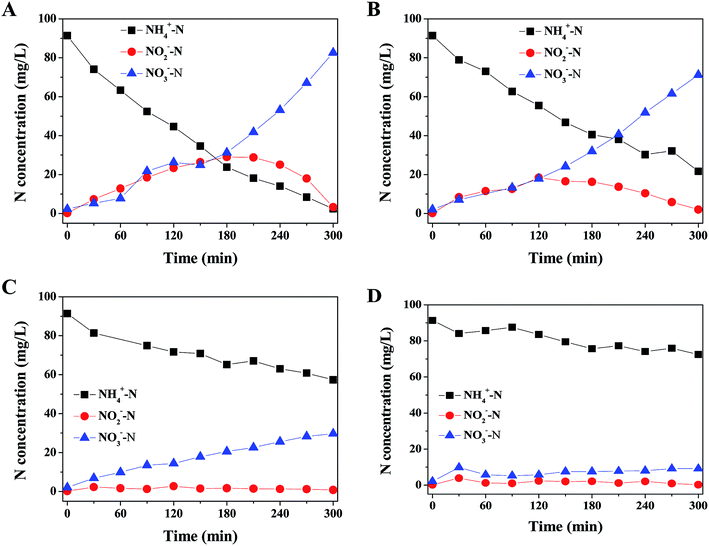 | ||
| Fig. 1 Effect of 2,4-DCP on the variations of nitrogen species: (A) 0 mg L−1; (B) 5 mg L−1; (C) 20 mg L−1; (D) 50 mg L−1. | ||
However, the effluent NH4+–N concentrations increased to 21.62, 57.30 and 72.43 mg L−1 with the exposure of 2,4-DCP at 5, 20 and 50 mg L−1, respectively, which were much higher than that of control experiment. As a result, the NH4+–N removal efficiency decreased to 20.71% in the presence of 2,4-DCP at 50 mg L−1. In addition, no nitrite accumulation were observed in the effluents, suggesting that 2,4-DCP had a relatively higher toxic effect on ammonia oxidizing bacteria (AOB) than nitrite oxidizing bacteria (NOB). The possible reason may be attributed to the structure of aerobic granular sludge, which has a unique granule attribute including heterotrophic, aerobic bacteria grown on the outside, ammonium-oxidizing bacteria in the middle, and anaerobic bacteria in the core of the granules.
The toxic effects of 2,4-DCP on biological nitrogen removal in many municipal and industrial wastewater treatment plants have been investigated in recent years. Kargi et al. evaluated the biological treatment of synthetic wastewater containing 2,4-DCP in an activated sludge unit, showing that the effluent 2,4-DCP increased with the increased of feed 2,4-DCP above 150 mg L−1.17 Chen et al. investigated the response of activated sludge to the presence of 2,4-DCP, implying that 2,4-DCP slightly reduced the specific oxygen uptake rate of activated sludge.18 Although it is more challenging to degrade of 2,4-DCP in aerobic system, aerobic granular sludge is able to retain a higher amount of chlorophenol-degrading bacteria, so as to achieve higher chlorophenol degradation activity and higher tolerance to chlorophenol toxicity.19 Wang et al. cultivated aerobic granules for 2,4-DCP biological degradation by using glucose as a co-substrate, suggesting that the potential application of aerobic granular sludge in the treatment of industrial wastewater containing chlorophenols and other inhibitory chemicals.19
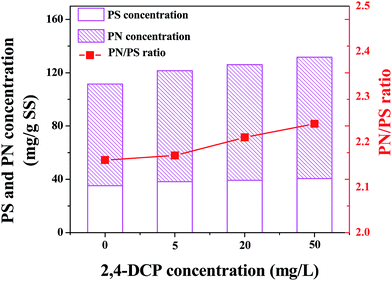
3.2 Toxicity of 2,4-DCP on EPS production
Fig. 2 shows the variations of EPS production after the exposure of different 2,4-DCP concentrations. In the control experiment, the PS and PN concentrations were 35.22 ± 0.69 and 76.28 ± 0.34, respectively. At the end of the short-term toxicity experiment, the PS and PN concentrations generally increased to 38.25 ± 1.46 and 83.30 ± 0.31 mg g−1 SS, respectively, implying that the presence of 2,4-DCP played an important role on the EPS production (p < 0.05). Moreover, the ratio of PN/PS increased from 2.16 to 2.24, indicating that the production of PN was more sensitive than PS in the expose of 2,4-DCP.It is well reported that EPS play an important role in the production of microorganisms against environmental pollutants shocks, including salt, heavy metal, nanoparticles, pigments/dyes etc.20 Wei et al. assessed toxicity of 4-chlorophenol to aerobic granular sludge and its interaction with EPS, indicating that an obviously increasing tendency of PS concentrations was observed.21 Shi et al. compared the response of nitrifying granules (NG) with conventional granules (CG) under tetracycline (TC) stress, showing the higher production of PN than that of PS from EPS to protect themselves from TC toxicity.22 The obtained EPS production trend in this study was in agreement with the above literatures.
3.3 3D-EEM
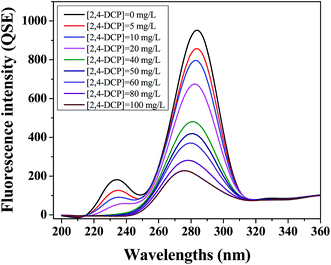
EPS play an important role in the field of wastewater treatment which directly contact and interact with toxic substance as the first barrier of microbial cells with their abundant surface binding sites. Therefore, it is essential to study the interaction and mechanism between EPS and toxic substance, in order to better understand the role of EPS in the removal of toxic substance in biological nitrogen removal system. In addition, the interaction between EPS and toxic substance is also significant to evaluate the stability of aerobic granular sludge system, due to its potential capacity affecting the chemical structure and key component of EPS.In view of this point, 3D-EEM spectroscopy was used, as a rapid, sensitive and useful technique, to evaluate the chemical characteristics and molecular features of EPS.23 Fig. 3 shows the 3D-EEM fluorescence spectra of EPS after the addition of various dosages of 2,4-DCP. The fluorescence parameters, including peak location, maximum intensity, and peak intensity, are listed in Table 1. As shown in Fig. 3, two main peaks were identified in the control EPS without the presence of 2,4-DCP. The first peak was identified at excitation/emission wavelengths (Ex/Em) of 285/356 nm (Peak A), while the second peak was identified at Ex/Em of 238/361 nm (Peak B), which were related to tryptophan-like protein and aromatic-like protein, respectively.24 The two peaks were similar with the literature reported by Sheng et al., in which EPS was extracted aerobic and anaerobic sludge.25
 | ||
| Fig. 3 3D-EEM fluorescence spectra of EPS after addition of various dosages of 2,4-DCP: (A) 0 mg L−1; (B) 20 mg L−1; (C) 50 mg L−1; (D) 80 mg L−1. | ||
| 2,4-DCP concentration (mg L−1) | Peaks | Ex/Em | Intensity |
|---|---|---|---|
| 0 | A | 285/356 | 495.6 |
| B | 238/361 | 270.7 | |
| 20 | A | 285/342 | 190.0 |
| 50 | A | 260/361 | 143.2 |
| 80 | A | 260/358 | 60.8 |
After the addition of different doses of 2,4-DCP, the intensities of peak A and peak B obviously decreased from 495.6 to 60.8 and 270.7 to 0, respectively, indicating that the fluorescence of EPS was strongly quenched by 2,4-DCP with different degrees. The phenomena was in agreement with the experimental results reported by Zhang et al., in which EPS was extracted from natural biofilm and interacted with Hg(II).26 It is noted from their results that both protein-like fluorescence peaks were indentified and significantly quenched.
3.4 Synchronous fluorescence spectra
Synchronous fluorescence spectroscopy can provide the information about tyrosine residues or tryptophan residues of protein, which is obtained through the simultaneous scanning of the excitation and emission spectrum by maintaining a constant wavelength interval (Δλ) at 15 or 60 nm.27 Fig. 4 shows the synchronous fluorescence spectra of the interaction between EPS and 2,4-DCP with Δλ at 60 nm. Results implied that the fluorescence intensities of EPS quenched from 952.0 to 227.3, indicating the strong interaction between 2,4-DCP and EPS. Furthermore, a slightly blue shift was observed to shorter wavelengths (from 285 to 276 nm), suggesting that the chemical structural changes of EPS after the addition of 2,4-DCP. The obtained results were in agreement with the literature reported by Zhang et al.,28 in which the fluorescent quenching of biofilm EPS with Cu(II) was investigated.3.5 Fluorescence quenching mechanism
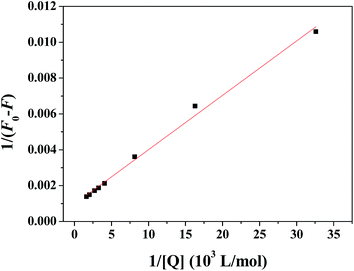
Dynamic and static quenching are the two main mechanisms for fluorescence quenching depending on the way of interaction between quencher and EPS.29 In order to better understand the quenching mechanism, the fluorescence quenching data were modeled with Stern–Volmer eqn (1):| F0/F = 1 + Ksv[2,4-DCP] = 1 + Kqτ0[2,4-DCP] | (1) |
As shown in Fig. 5, there was good linear relationship (R2 = 0.9843) between F0/F − 1 and [2,4-DCP]. And the quenching rate constant of Kq is calculated as 5.22 × 1011 L mol−1 S−1. In general, the maximum scatter collision quenching constant of quencher to biomacromolecule is 2.0 × 1010 L mol−1 s−1.30 As the data show, the obtained rate constant is much greater than Kq of scatter procedure. Therefore, static fluorescence quenching may be the main quenching mechanism between EPS and 2,4-DCP.
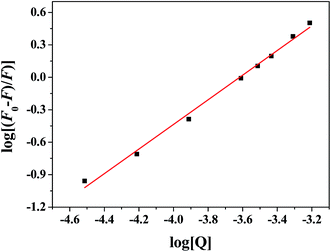
The modified Stern–Volmer eqn (2) was used to evaluate the static fluorescence quenching process:
 | (2) |
For static fluorescence quenching process, fluorescence intensity data could be further used to determine the binding constant (Kb) and the number of binding sites (n) for the complex, as expressed by the following eqn (3).
 | (3) |
3.6 FTIR
The functional groups of EPS samples before and after the addition of 2,4-DCP were measured by FTIR spectroscopy, as shown in Fig. 8. Data show that functional groups of the two samples were similar but with small changes. A broad peak around 3418 cm−1 was observed and related to O–H group of carbohydrates. A weak peak at 2930 cm−1 was assigned to C–H stretching vibration of the aliphatic CH2 group, which represents the presence of organic substance in protein.31,32 The presence of an asymmetric medium stretching peak at 1630 cm−1 may corresponded to the ring stretching of mannose or galactose.33 Another peak at 1407 cm−1 could be attributed to the symmetric stretching of the –COO− group. The absorption peaks ranging from 1000–1200 cm−1 were designated to C–O–O and C–O, which indicated the occurrence of carbohydrates.34 The main hydrophily of EPS was attributed to the presence of O–H group, while the presence of aliphatic CH2 group was responsible for the hydrophobic.354. Conclusions
The performance of biological nitrogen removal was assessed in aerobic granular sludge process in the exposure of toxic 2,4-DCP treating ammonia rich wastewater. The contents of EPS including PS and PN increased in the presence of 2,4-DCP. A strong interaction between EPS and 2,4-DCP was characterized by using a combined 3D-EEM fluorescence spectroscopy and synchronous fluorescence spectra. The obtained results implied that the toxicity of 2,4-DCP played an important role in the production and interaction with EPS in aerobic granular sludge system.Acknowledgements
This study was supported by the Natural Science Foundation of Chinese Province (21377046), Special project of independent innovation and achievements transformation of Shandong Province (2014ZZCX05101), Science and technology development plan project of Shandong province (2014GGH217006), and QW thanks the Special Foundation for Taishan Scholar Professorship of Shandong Province and UJN (no. ts20130937).References
- Y. J. Chan, M. F. Chong, C. L. Law and D. G. Hassell, Chem. Eng. J., 2009, 155, 1–18 CrossRef CAS PubMed.
- L. Zhu, Y. Yu, X. Dai, X. Xu and H. Qi, Chem. Eng. J., 2013, 217, 442–446 CrossRef CAS PubMed.
- D. Wei, X. D. Xue, S. W. Chen, Y. F. Zhang, L. G. Yan, Q. Wei and B. Du, Appl. Microbiol. Biotechnol., 2013, 97, 9235–9243 CrossRef CAS PubMed.
- Y. Q. Liu, Y. Liu and J. H. Tay, Appl. Microbiol. Biotechnol., 2004, 65, 143–148 CrossRef CAS PubMed.
- L. Zhu, H. Y. Qi, M. L. Lv, Y. Kong, Y. W. Yu and X. Y. Xu, Bioresour. Technol., 2012, 124, 455–459 CrossRef CAS PubMed.
- D. Wei, L. Shi, T. Yan, G. Zhang, Y. F. Wang and B. Du, Bioresour. Technol., 2014, 171, 211–216 CrossRef CAS PubMed.
- G. P. Sheng, H. Q. Yu and X. Y. Li, Biotechnol. Adv., 2001, 28, 882–894 CrossRef PubMed.
- G. Ricco, M. C. Tomei, R. Ramadori and G. Laera, Water Res., 2004, 38, 2103–2110 CrossRef CAS PubMed.
- S. Comte, G. Guibaud and M. Baudu, J. Hazard. Mater., 2008, 151, 185–193 CrossRef CAS PubMed.
- H. E. Ensley, J. T. Barber, M. A. Polito and A. I. Oliver, Environ. Toxicol. Chem., 1994, 13, 325–331 CrossRef CAS.
- J. H. Tay, Q. S. Liu and Y. Liu, Environ. Technol., 2002, 23, 931–936 CrossRef CAS PubMed.
- X. Zheng, R. Wu and Y. Chen, Environ. Sci. Technol., 2011, 45, 2826–2832 CrossRef CAS PubMed.
- X. Y. Li and S. F. Yang, Water Res., 2007, 41, 1022–1030 CrossRef CAS PubMed.
- E. Larson, B. Howlett and A. Jagendorf, Anal. Biochem., 1986, 155, 243–248 CrossRef CAS.
- APHA, AWWA, WEF, 21st ed, Washington, D.C. 2005.
- D. C. Peng, N. Bernet, J. P. Delgenes and R. Moletta, Water Res., 1999, 33, 890–893 CrossRef CAS.
- F. Kargi, S. Eker and A. Uygur, J. Environ. Manage., 2005, 76, 191–196 CrossRef CAS PubMed.
- G. W. Chen, H. Q. Yu, H. X. Liu and D. Q. Xu, Process Biochem., 2006, 41, 1758–1763 CrossRef CAS PubMed.
- S. G. Wang, X. W. Liu, H. Y. Zhang, W. X. Gong, X. F. Sun and B. Y. Gao, Chemosphere, 2007, 69, 769–775 CrossRef CAS PubMed.
- S. Q. Ni, J. Ni, N. Yang and J. Wang, Bioresour. Technol., 2013, 143, 555–561 CrossRef CAS PubMed.
- D. Wei, Y. F. Wang, X. D. Wang, M. T. Li, F. Han, L. Y. Ju, G. Zhang, L. Shi, K. Li, B. F. Wang, B. Du and Q. Wei, J. Hazard. Mater., 2015, 289, 101–107 CrossRef CAS PubMed.
- Y. J. Shi, S. F. Xing, X. H. Wang and S. G. Wang, Bioresour. Technol., 2013, 139, 170–175 CrossRef CAS PubMed.
- M. Fukushima, K. Tatsumi and S. Nagao, Environ. Sci. Technol., 2001, 35, 3683–3690 CrossRef CAS.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Environ. Sci. Technol., 2003, 37, 5701–5710 CrossRef CAS.
- G. P. Sheng and H. Q. Yu, Water Res., 2006, 40, 1233–1239 CrossRef CAS PubMed.
- D. Y. Zhang, X. L. Pan, K. M. Mostofa, X. Chen, G. J. Mu, F. C. Wu, J. Liu, W. J. Song, J. Y. Yang, Y. L. Liu and Q. L. Fu, J. Hazard. Mater., 2010, 175, 359–365 CrossRef CAS PubMed.
- Y. Q. Wang, H. M. Zhang, G. C. Zhang, S. X. Liu, Q. H. Zhou, Z. H. Fei and Z. T. Liu, Int. J. Biol. Macromol., 2007, 41, 243–250 CrossRef CAS PubMed.
- D. Zhang, D. J. Lee and X. Pan, J. Taiwan Inst. Chem. Eng., 2012, 43, 450–454 CrossRef CAS PubMed.
- Y. J. Hu, Y. Liu, L. X. Zhang, R. M. Zhao and S. S. Qu, J. Mol. Struct., 2005, 750, 174–178 CrossRef CAS PubMed.
- C. Q. Jiang, M. X. Gao and J. X. He, Anal. Chim. Acta, 2002, 452, 185–189 CrossRef CAS.
- K. Kavita, A. Mishra and B. Jha, Carbohydr. Polym., 2013, 94, 882–888 CrossRef CAS PubMed.
- S. S. Ahluwalia and D. Goyal, Eng. Life Sci., 2005, 5, 158–162 CrossRef CAS.
- F. Freitas, V. D. Alves, M. Carvalheira, N. Costa, R. Oliveira and M. A. Reis, Carbohydr. Polym., 2009, 78, 549–556 CrossRef CAS PubMed.
- P. Bramhachari and S. Dubey, Lett. Appl. Microbiol., 2006, 43, 571–577 CrossRef CAS PubMed.
- T. Karbowiak, E. Ferret, F. Debeaufort, A. Voilley and P. Cayot, J. Membr. Sci., 2011, 370, 82–90 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |