Folded three-dimensional graphene with uniformly distributed mesopores for high-performance supercapacitors†
Abstract
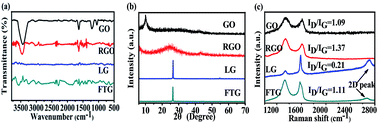
Folded three-dimensional graphene (FTG) is prepared through self-assembly of graphite oxide (GO) and liquid-phase exfoliation graphene (LG), followed by sonication and reduction processes. The obtained FTG consists of few-layer graphene. And it possesses a high degree of crystallinity, three-dimensional architecture and uniformly distributed mesopores. A supercapacitor based on an FTG electrode exhibits enhanced electrochemical performance. The FTG electrode exhibits high specific capacitance of 195.4 F g−1 at a scan rate of 1 mV s−1 and excellent cycling stability with 93.9% of its initial capacitance at a large scan rate of 500 mV s−1 after 8000 cycles. The supercapacitor fabricated with the FTG electrode delivers a high energy density of 27.1 W h kg−1 at a power density of 97.7 W kg−1. These results suggest that FTG is a promising material for high-performance supercapacitor applications.

 Please wait while we load your content...
Please wait while we load your content...