Metal-free iodine-mediated synthesis of vinyl sulfones at room temperature using water as solvent†
Abstract
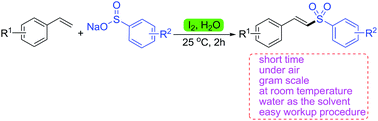
Metal-free efficient iodine-mediated synthesis of vinyl sulfones utilizing aryl sulfinates and alkenes has been realized under mild conditions in water. Notably, sodium methanesulfinate was used in this transformation, affording the β-iodo sulfones in good yields. This simple, efficient and environmentally benign transformation provides an attractive approach to various vinyl sulfones or β-iodo sulfones.

 Please wait while we load your content...
Please wait while we load your content...