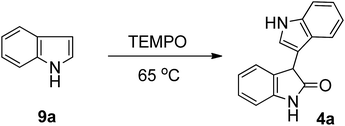
Silver-catalyzed TEMPO oxidative homocoupling of indoles for the synthesis of 3,3′-biindolin-2-ones†
Feng Lin,
Yu Chen,
Baoshuang Wang,
Wenbing Qin* and
Liangxian Liu*
Key Laboratory of Organo-Pharmaceutical Chemistry of Jiangxi Province, Gannan Normal University, Ganzhou 341000, PR China. E-mail: lxliu@xmu.edu.cn
First published on 13th April 2015
Abstract
An oxidative homo dimerization of free indole derivatives, by means of silver catalysis and TEMPO oxidant, was first successful demonstrated. This new methodology is both atom and step efficient and is applicable to a broad scope of substrates, allowing the synthesis of a range of synthetically valuable substituted C3–C3′ bisindolin-2-ones in moderate to excellent yields.
Introduction
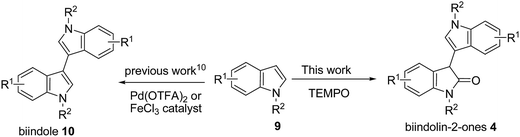
Indole derivatives are important compounds widespread in nature that exhibit significant biological activity and possess a privileged structure in drug discovery.1 Many substituted C3–C3′ bisindole alkaloids have attracted considerable attention in view of their potent biological activities.2 For example, bisindigotin (1) was isolated from the Chinese medicinal herb Isatis indigotica. This herbal plant has long been used as a folk medicine in China for treatment of viral diseases and diseases with inflammatory nature.3In addition, oxindole framework is a privileged heterocyclic motif that constructs the core of a large family of bioactive alkaloids and a series of pharmaceutically active compounds.4 Moreover, substituted C3–C3′ bisindolin-2-ones (4) are a versatile structural unit for the preparation of other important pharmaceuticals and natural products5,6 such as chimonanthine (2a) and folicanthine (2b) (Fig. 1).7 For instance, Trost and co-workers reported the synthesis of bisindolin-2-one 8 from 5 in three steps via 7 (Scheme 1).7c,d Thus, it is highly desirable to develop straightforward and highly efficient methods for the construction of substituted C3–C3′ biindolyl scaffolds. Both chemical and enzymatic synthetic methods have been reported for the construction of biindolyls.8–11 However, most of these procedures require the prefunctionalization of indoles to their halide or metallic derivatives, or using expensive metal catalysts and high loading of metal oxidants. In light of the recent demands for mild, environmentally benign and atom economic organic syntheses, much attention has been paid to the direct C–C bond formation of indoles (Scheme 2). As part of our ongoing project aimed at developing selective and environmentally benign chemical transformations,12 we studied the TEMPO-catalyzed oxidative homocoupling of indoles. Herein, we report the first successful example of silver-catalyzed TEMPO oxidative homocoupling of indoles affording substituted C3–C3′ bisindolin-2-ones (4). To the best of our knowledge, this work represents the first example of one-step synthesis of bisindolin-2-ones via direct C–H transformation.
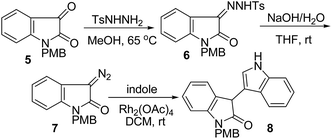 | ||
| Scheme 1 Known procedure: 3 steps, 38% overall yield.7c | ||
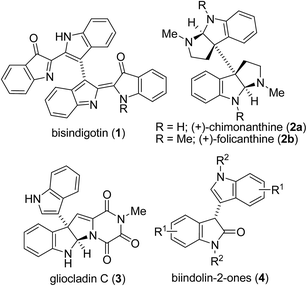 | ||
| Fig. 1 Representative examples of cytotoxic and antibiotic C3–C3′ bisindole alkaloids and important intermediates (4). | ||
Results and discussion
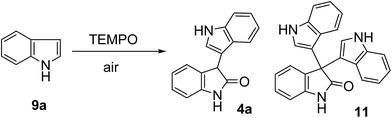
We started our model reaction by investigating indole 9a for the optimization of reaction conditions (Table 1). 3,3′-Dimer 4a was obtained in 22% isolated yield upon treatment of 9a with 10 mol% BiCl3 in pyridine at 65 °C for 48 h under an air atmosphere. However, we also observed 13% of trimeric byproduct 11 (Table 1, entry 7). Other metal salts, such as FeCl3·6H2O, InCl3·4H2O, SnCl4·5H2O, and ZrCl4, showed no reactivity (Table 1, entries 1–4). ZnCl2 and CoCl2 were found to be less efficient (Table 1, entries 5 and 6). To our delight, 4a was obtained in 45% isolated yield in the presence of 10 mol% AgNO3 (Table 1, entry 8). Among the silver sources used, AgNO3 exhibited the highest catalytic reactivity (Table 1, entries 9–11). Further investigations revealed that there was obvious loss in yield when catalyst loading was reduced to 5 mol% (Table 1, entry 12). However, increasing the loading of AgNO3 to 20 mol% in the reaction boosted the yield of 4a to 61% (Table 1, entry 14).| Entry | Catalyst (mol%) | TEMPO (mmol) | Acid (mmol) | Yieldb (%) (4a/11) |
|---|---|---|---|---|
| a Condition: 9a (0.5 mmol), pyridine (1 mL), 65 °C, 48 h, under open air.b Isolated yields. | ||||
| 1 | FeCl3·6H2O (10) | 0.2 | PhCO2H (0.15) | 0/0 |
| 2 | InCl3·4H2O (10) | 0.2 | PhCO2H (0.15) | 0/0 |
| 3 | SnCl4·4H2O (10) | 0.2 | PhCO2H (0.15) | 0/0 |
| 4 | ZrCl4 (10) | 0.2 | PhCO2H (0.15) | 0/0 |
| 5 | ZnCl2 (10) | 0.2 | PhCO2H (0.15) | 5/13 |
| 6 | CoCl2 (10) | 0.2 | PhCO2H (0.15) | 17/16 |
| 7 | BiCl3 (10) | 0.2 | PhCO2H (0.15) | 22/13 |
| 8 | AgNO3 (10) | 0.2 | PhCO2H (0.15) | 45/trace |
| 9 | Ag2CO3 (10) | 0.2 | PhCO2H (0.15) | 31/0 |
| 10 | AgOTf (10) | 0.2 | PhCO2H (0.15) | 27/0 |
| 11 | AgBF4 (10) | 0.2 | PhCO2H (0.15) | 40/0 |
| 12 | AgNO3 (5) | 0.2 | PhCO2H (0.15) | 16/0 |
| 13 | AgNO3 (15) | 0.2 | PhCO2H (0.15) | 52/0 |
| 14 | AgNO3 (20) | 0.2 | PhCO2H (0.15) | 61/0 |
| 15 | AgNO3 (25) | 0.2 | PhCO2H (0.15) | 60/0 |
| 16 | AgNO3 (20) | — | PhCO2H (0.15) | 0/0 |
| 17 | AgNO3 (20) | 0.1 | PhCO2H (0.15) | 10/0 |
| 18 | AgNO3 (20) | 0.25 | PhCO2H (0.15) | 48/0 |
| 19 | AgNO3 (20) | 0.3 | PhCO2H (0.15) | 65/0 |
| 20 | AgNO3 (20) | 0.35 | PhCO2H (0.15) | 69/0 |
| 21 | AgNO3 (20) | 0.4 | PhCO2H (0.15) | 60/0 |
| 22 | AgNO3 (20) | 0.35 | CH3SO3H (0.15) | 0/45 |
| 23 | AgNO3 (20) | 0.35 | TsOH (0.15) | 0/56 |
| 24 | AgNO3 (20) | 0.35 | HOAc (0.15) | 0/23 |
| 25 | AgNO3 (20) | 0.35 | PhCO2H (0.05) | 39/0 |
| 26 | AgNO3 (20) | 0.35 | PhCO2H (0.1) | 73/0 |
| 27 | AgNO3 (20) | 0.35 | PhCO2H (0.2) | 65/0 |
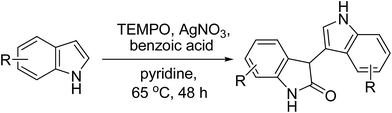
It was also found that the loading amounts of TEMPO are critical to the reaction. In the absence of TEMPO, the dimerization reaction did not proceed (Table 1, entry 16). An oxidant loading of 70 mol% was found to be optimal as decreasing the oxidant loading to 60 mol%, 50 mol% or 20 mol% led to the reduced yield of 65%, 48% and 10%, respectively (Table 1, entries 17–19). The modification to the acids indicated that benzoic acid was identified as the most suitable acid for the formation of 4a. For example, 73% yield of dimerization product was obtained when using 0.20 equiv. of benzoic acid (Table 1, entry 26). Interestingly, other acid such as CH3SO3H, TsOH, and HOAc proved to be inefficient for the formation of 4a (Table 1, entries 22–24). The type of solvent was vital to the present coupling reaction. Pyridine was found to be the best choice (ESI†). Taken together, we concluded that the optimized conditions were using 20 mol% AgNO3 as the catalyst, 70 mol% TEMPO as the oxidant, PhCO2H as the acid, pyridine as the solvent, and carrying out the reaction at 65 °C for 48 h.
With the optimized conditions in hand, we then set out to explore the scope and limitations of the oxidative homocoupling of indoles, and the results are summarized in Table 2. The reaction can tolerate a variety of functional groups at the 4, 5, 6, and 7 positions of indoles, such as F, Cl, Br, CH3, CH3O, BnO, CN, and CO2CH3. The substituent effect on the indole ring was then investigated. The results have shown that electronegativities of the substituents played a major role in governing the reactivity of the substrates. Electron-donating substituents showed better results than electron-withdrawing substituents in this transformation. For example, 7-methyl-1H-indole was transformed into 4n in 90% yield (Table 2, entry 14). Substrates with strong electron-withdrawing substituents at C5-position, such as NO2, disfavored the transformation (Table 2, entry 8). It is worth noting that 4-methoxyindole gave 4-methoxyisatin 12 in 56% yield under the present conditions (Table 2, entry 17).
| Entry | R | Product | Yieldb [%] |
|---|---|---|---|
| a Reaction conditions: indole (0.5 mmol), benzoic acid (0.1 mmol), TEMPO (0.35 mmol), pyridine (1 mL), 65 °C.b Isolated yield.c Yield of 4-methoxyisatin. | |||
| 1 | H | 4a | 73 |
| 2 | 5-F | 4b | 62 |
| 3 | 5-Br | 4c | 71 |
| 4 | 5-CH3 | 4d | 84 |
| 5 | 5-OBn | 4e | 75 |
| 6 | 5-CN | 4f | 37 |
| 7 | 5-CO2CH3 | 4g | 70 |
| 8 | 5-NO2 | 4h | 0 |
| 9 | 6-F | 4i | 58 |
| 10 | 6-Cl | 4j | 75 |
| 11 | 6-OBn | 4k | 77 |
| 12 | 6-CO2CH3 | 4l | 71 |
| 13 | 7-Cl | 4m | 59 |
| 14 | 7-CH3 | 4n | 90 |
| 15 | 7-OCH3 | 4o | 72 |
| 16 | 7-OBn | 4p | 73 |
| 17 | 4-OCH3 | 12 | 56c |
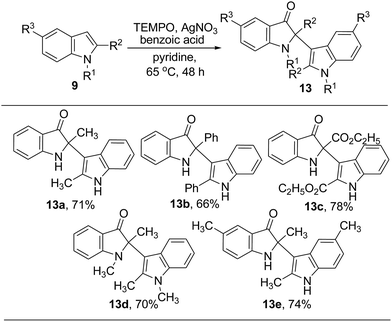
In addition, the reaction was applicable to other indoles including 2-methyl-1H-indole 9q, 2-phenyl-1H-indole 9r, ethyl-1H-indole-2-carboxylate 9s, 1,2-dimethyl-1H-indole 9t, 2,5-dimethyl-1H-indole 9u. New cross dimerized products 13 with the unique 2,3′-linkage were obtained in good yields when 9 was treated with 20 mol% AgNO3, 70 mol% TEMPO in the presence of benzoic acid in pyridine at 65 °C for 48 h (Scheme 3).
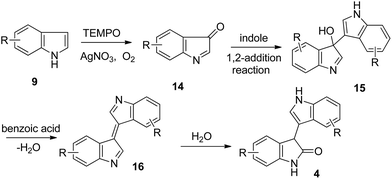
Because the TEMPO oxidative coupling was performed in air, the role of O2 in this reaction was explored by conducting several control experiments. Under an O2 atmosphere, the reaction yield was not increased, but a more rapid conversion of the starting material to the reaction product was observed by TLC detection compared to that performed under air conditions. However, only trace amount of the product was obtained under an Ar atmosphere, even a long reaction time (Table 3, entry 2). These results indicated that O2 is essential for the TEMPO oxidative homocoupling. In addition, no homocoupling product was detectable when indole was reacted in the absence of benzoic acid (Table 3, entry 3). Furthermore, the corresponding product 4a was obtained in 25% yield when indole was reacted in the absence of AgNO3 (Table 3, entry 4), indicating that AgNO3 is crucial for the oxidative homocoupling.
Based on the previous studies13 and our experimental results, a plausible reaction path was outlined in Scheme 4. First, indole was oxidized in the presence of TEMPO, AgNO3 and O2 into indenone 14 which should be unstable and was never isolated.13a Then, a rapid nucleophilic addition of another indole molecule on this intermediate followed by dehydration in the presence of benzoic acid affords intermediate 16, which should be unstable.13b Finally, intermediate 16 reacted rapidly with H2O to give product 4.
Conclusions
In conclusion, we have demonstrated the first example of silver-catalyzed TEMPO oxidative homo dimerization of free indole derivatives leading to C3–C3′ bisindolin-2-ones 4 in moderate to excellent yields with high regioselectivity. We were very pleased to find that the C2–C3′ bisindolin-3-ones 13 can be obtained when 2-substituted indoles were subjected to the standard procedures. The advantages of this new method include the broad substrate scope, operational simplicity, high atom-economy, and the use of inexpensive AgNO3 as the catalyst. Moreover, the high halogen compatibility of the process can provide a facile access to halo-substituted bisindolin-2-ones.Acknowledgements
The authors are grateful to the NSF of China (nos 21162001, 21202023, and 21462002), Natural Science Foundation of Jiangxi Province (no. 20132BAB203007) and Jiangxi Province Office of Education Support Program (no. GJJ13666) for financial support.References
- (a) R. J. Sundberg, Indoles, Academic Press, San Diego, 1996 Search PubMed; (b) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873–2920 CrossRef CAS PubMed; (c) L. Joucla and L. Djakovitch, Adv. Synth. Catal., 2009, 351, 673–714 CrossRef CAS PubMed; (d) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608–9644 CrossRef CAS PubMed; (e) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449–4465 RSC; (f) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873–2920 CrossRef CAS PubMed.
- (a) S. Tadano, Y. Mukaeda and H. Ishikawa, Angew. Chem., Int. Ed., 2013, 52, 7990–7994 CrossRef CAS PubMed; (b) L. Furst, J. M. R. Narayanam and C. R. J. Stephenson, Angew. Chem., Int. Ed., 2011, 50, 9655–9659 CrossRef CAS PubMed; (c) K. Foo, T. Newhouse, I. Mori, H. Takayama and P. S. Baran, Angew. Chem., Int. Ed., 2011, 50, 2716–2719 CrossRef CAS PubMed; (d) L.-Y. Li, D.-H. Li, Y.-P. Luan, Q.-Q. Gu and T.-J. Zhu, J. Nat. Prod., 2012, 75, 920–927 CrossRef CAS PubMed; (e) A. A. EI-Gamal, W.-L. Wang and C.-Y. Duh, J. Nat. Prod., 2005, 68, 815–817 CrossRef PubMed; (f) C.-J. Zheng, C.-J. Kim, K. S. Bae, Y.-H. Kim and W.-G. Kim, J. Nat. Prod., 2006, 69, 1816–1819 CrossRef CAS PubMed.
- X.-Y. Wei, C.-Y. Leung, C. K. C. Wong, X.-L. Shen, R. N. S. Wong, Z.-W. Cai and N.-K. Mak, J. Nat. Prod., 2005, 68, 427–429 CrossRef CAS PubMed.
- For reviews, please see: (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (b) H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36–51 CrossRef CAS PubMed; (c) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209–2219 CrossRef CAS PubMed.
- For reviews, please see: (a) L. Hong and R. Wang, Adv. Synth. Catal., 2013, 355, 1023–1052 CrossRef CAS PubMed; (b) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS PubMed.
- For selected examples, please see: (a) Y.-J. Zhao, L. Liu, W. Sun, J.-F. Lu, D. McEachern, X.-Q. Li, S.-H. Yu, D. Bernard, P. Ochsenbein, V. Ferey, J.-C. Carry, J. R. Deschamps, D.-X. Sun and S.-M. Wang, J. Am. Chem. Soc., 2013, 135, 7223–7234 CrossRef CAS PubMed; (b) Z.-Y. Cao, X.-M. Wang, C. Tan, X.-L. Zhao, J. Zhou and K.-L. Ding, J. Am. Chem. Soc., 2013, 135, 8197–8200 CrossRef CAS PubMed; (c) F. Manoni and S. J. Connon, Angew. Chem., Int. Ed., 2014, 53, 2628–2632 CrossRef CAS PubMed; (d) Z. Lian, S. D. Friis and T. Skrydstrup, Angew. Chem., Int. Ed., 2014, 53, 9582–9586 CrossRef CAS PubMed; (e) H.-F. Zheng, P. He, Y.-B. Liu, Y.-L. Zhang, X.-H. Liu, L.-L. Lin and X.-M. Feng, Chem. Commun., 2014, 50, 8794–8796 RSC; (f) F.-L. Hu, Y. Wei and M. Shi, Chem. Commun., 2014, 50, 8912–8914 RSC; (g) F. Shi, R.-Y. Zhu, W. Dai, C.-S. Wang and S.-J. Tu, Chem. – Eur. J., 2014, 20, 2597–2604 CrossRef CAS PubMed; (h) F. Tan, L.-Q. Lu, Q.-Q. Yang, W. Guo, Q. Bian, J.-R. Chen and W.-J. Xiao, Chem. – Eur. J., 2014, 20, 3415–3420 CrossRef CAS PubMed; (i) X. Li, M.-H. Lin, Y. Han, F. Wang and J.-P. Cheng, Org. Lett., 2014, 16, 114–117 CrossRef CAS PubMed; (j) K. Suman, L. Srinu and S. Thennarasu, Org. Lett., 2014, 16, 3732–3735 CrossRef CAS PubMed.
- (a) H. Mitsunuma, M. Shibasaki, M. Kanai and S. Matsunaga, Angew. Chem., Int. Ed., 2012, 51, 5217–5221 CrossRef CAS PubMed; (b) C. Guo, J. Song, J.-Z. Huang, P.-H. Chen, S.-W. Luo and L.-Z. Gong, Angew. Chem., Int. Ed., 2012, 51, 1046–1050 CrossRef CAS PubMed; (c) B. M. Trost, J. Xie and J. D. Sieber, J. Am. Chem. Soc., 2011, 133, 20611–20622 CrossRef CAS PubMed; (d) C. Marti and E. M. Carreira, J. Am. Chem. Soc., 2005, 127, 11505–11515 CrossRef CAS PubMed.
- For the construction of C2–C2′ biindolyl, please see: (a) A. Pezzella, L. Panzella, O. Crescenzi, A. Napolitano, S. Navaratman, R. Edge, E. J. Land, V. Barone and M. d'lschia, J. Am. Chem. Soc., 2006, 128, 15490–15498 CrossRef CAS PubMed; (b) P. A. Keller, N. R. Yepuri, M. J. Kelso, M. Mariani, B. W. Skelton and A. H. White, Tetrahedron, 2008, 64, 7787–7795 CrossRef CAS PubMed; (c) X.-H. Xu, G.-K. Liu, A. Azuma, E. Tokunaga and N. Shibata, Org. Lett., 2011, 13, 4854–4857 CrossRef CAS PubMed; (d) S. Liu and X.-J. Hao, Tetrahedron Lett., 2011, 52, 5640–5642 CrossRef CAS PubMed; (e) L. Panzella, A. Pezzella, A. Napolitano and M. d'lschia, Org. Lett., 2007, 9, 1411–1414 CrossRef CAS PubMed; (f) C. A. Meric, Y. You, D. M. McInnes, A. L. Zechman, M. M. Miller and Q.-L. Deng, Tetrahedron, 2001, 57, 5199–5212 CrossRef; (g) W.-J. Zhang, Z. Liu, S.-M. Li, T.-T. Yang, Q.-B. Zhang, L. Ma, X.-P. Tian, H.-B. Zhang, C.-G. Huang, S. Zhang, J.-H. Ju, Y.-M. Shen and C.-S. Zhang, Org. Lett., 2012, 14, 3364–3367 CrossRef CAS PubMed; (h) N. R. Yepuri, R. Haritakul, P. A. Keller, B. W. Skelton and A. H. White, Tetrahedron Lett., 2009, 50, 2501–2504 CrossRef CAS PubMed.
- For the construction of C2–C3′ biindolyl, please see: (a) A. Pezzella, L. Panzella, A. Natangelo, M. Arzillo, A. Napolitano and M. d'Ischia, J. Org. Chem., 2007, 72, 9225–9230 CrossRef CAS PubMed; (b) Z.-J. Liang, J.-L. Zhao and Y.-H. Zhang, J. Org. Chem., 2010, 75, 170–177 CrossRef CAS PubMed; (c) Y.-X. Li, K.-G. Ji, H.-X. Wang, S. Ali and Y.-M. Liang, J. Org. Chem., 2011, 76, 744–747 CrossRef CAS PubMed.
- For the construction of C3–C3′ biindolyl, please see: (a) Y. Li, W.-H. Wang, S.-D. Yang, B.-J. Li, C. Feng and Z.-J. Shi, Chem. Commun., 2010, 46, 4553–4555 RSC; (b) T.-M. Niu and Y.-H. Zhang, Tetrahedron Lett., 2010, 51, 6847–6851 CrossRef CAS PubMed.
- A. Pezzella, D. Vogna and G. Prota, Tetrahedron: Asymmetry, 2003, 14, 1133–1140 CrossRef CAS.
- (a) Z.-W. Chen, M.-T. Luo, Y.-L. Wen, G.-T. Luo and L.-X. Liu, Org. Lett., 2014, 16, 3020–3023 CrossRef CAS PubMed; (b) W.-B. Qin, Q. Chang, Y.-H. Bao, N. Wang, Z.-W. Chen and L.-X. Liu, Org. Biomol. Chem., 2012, 10, 8814–8821 RSC; (c) Y.-H. Bao, J.-Y. Zhu, W.-B. Qin, Y.-B. Kong, Z.-W. Chen, S.-B. Tang and L.-X. Liu, Org. Biomol. Chem., 2013, 11, 7938–7945 RSC; (d) W.-B. Qin, J.-Y. Zhu, Y.-B. Kong, Y.-H. Bao, Z.-W. Chen and L.-X. Liu, Org. Biomol. Chem., 2014, 12, 4252–4259 RSC; (e) W.-B. Qin, Q. Chang, H.-Q. Luo, Y.-H. Bao, Z.-W. Chen and L.-X. Liu, Curr. Org. Synth., 2013, 10, 492–499 CrossRef CAS.
- (a) C. Ganachaud, V. Garfagnoli, T. Tron and G. Iacazio, Tetrahedron Lett., 2008, 49, 2476–2478 CrossRef CAS PubMed; (b) J. Bergman, S. Bergman and J. O. Lindström, Tetrahedron Lett., 1998, 39, 4119–4122 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and NMR data of the prepared compounds. See DOI: 10.1039/c5ra04106f |
| This journal is © The Royal Society of Chemistry 2015 |