Biomagnification of mercury in mollusks from coastal areas of the Chinese Bohai Sea†
Abstract
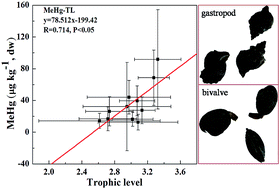
The multiple recognized mollusk species are usually regarded as one group lying at the second trophic level in the marine ecosystem. As a result, the virtual resolution of Hg uptake and transfer processes that occur in different mollusks would be overlooked. In this work, the concentrations of total mercury (THg) and methylmercury (MeHg), δ15N, δ13C and lipid contents were comprehensively analyzed in 11 mollusk species collected from the Chinese Bohai Sea during 2007–2012. The contents of THg and MeHg were in the range 27.2–461.1 and 2.1–295.5 μg kg−1, respectively. The trophic levels (TLs) were in the range 1.99–4.02. The biomagnification of Hg was evident from the significant positive correlations between Hg contents and TLs, and from the trophic magnification factors (TMFs). MeHg is the main species of Hg magnification in mollusks, while growth dilution occurs in the trophic transfer of inorganic mercury (IHg). TLs showed a greater effect on Hg levels in mollusks than lipid contents.

 Please wait while we load your content...
Please wait while we load your content...