Production of 5-hydroxymethylfurfural from agarose by using a solid acid catalyst in dimethyl sulfoxide†
Sang Jin Oha,
Juyi Parka,
Jeong Geol Nab,
You Kwan Ohb and
Yong Keun Chang*a
aDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology, Daejeon, 305-701, Korea. E-mail: ychang@kaist.ac.kr; Fax: +82-42-350-3910; Tel: +82-42-350-3927
bKorea Institute of Energy Research, Daejeon 305-343, Korea
First published on 15th May 2015
Abstract
In this study, an effective method for 5-HMF production from agarose, a biomass material derived from red-algae, is proposed. 5-HMF was produced from the decomposition of agarose by the catalytic action of a solid acid, Amberlyst 36, in dimethyl sulfoxide (DMSO), which is a polar aprotic solvent apt for providing a micro-aqueous environment. The moisture content in DMSO was found to be the governing factor for 5-HMF production, with an optimum level of 7.2% at which the 5-HMF yield was 62%, the highest level ever observed in the production of 5-HMF from agarose. DMSO was also found to protect 5-HMF, once produced, from rehydration in the present system. It was demonstrated that Amberlyst 36 could be repeatedly used in a packed-bed reactor, which was the prime objective of using a solid acid instead of a liquid acid as catalyst.
1. Introduction
The chemical and energy industries nowadays heavily depend on fossil resources such as oil, natural gas and coal. However, these resources pose the serious problems of greenhouse gas emission during their conversion to fuel or chemicals and in the life cycle of these products, as well as their expected depletion in the long run. For these reasons, an alternative renewable resource with no or much reduced net greenhouse emission is needed in many areas of the chemical and energy industries.1–4 Biomass is a promising candidate for such a purpose; it can be converted to various chemicals and energy materials through chemical, biological, and/or enzymatic processes.55-Hydroxymethylfurfural (5-HMF), a furan derivative, is called a ‘sleeping giant’ with many potential applications. It can be produced from biomass-derived carbohydrates and has good potential to be a sustainable substitute for petroleum-based building blocks used in the production of fine chemicals, pharmaceuticals and bio-fuels.6–8 Recently, many researchers have been interested in producing platform compounds, including 5-HMF, from biomass.
Since the discovery of 5-HMF at the end of the 19th century, many workers have tried to produce it from various raw materials.9 Biomass-derived monosaccharides such as fructose and glucose have been the most commonly studied precursors for the production of 5-HMF by dehydration. However, it has been found difficult to convert glucose, with its aldose configuration, to 5-HMF. On the contrary, fructose, a ketose, is readily converted to 5-HMF with high selectivity.10 For this reason fructose has played a role as a major material source for 5-HMF production for decades. Fructose, however, cannot be a viable source of 5-HMF synthesis due to its relatively low abundance in nature and high price.
There are emerging interests in producing 5-HMF from polysaccharides these days. Starch,11 cellulose,12–15 inulin,16–19 and cotton seed hull20 have been reported to be used as raw material for 5-HMF production. There is, however, a serious moral issue with starch and inulin, of consuming a food resource for the production of chemicals.21 Another candidate as material source for 5-HMF production is agarose, a galactan, contained in red algae. Agarose comprises over 50% of Gelidium amansii,22,23 one of the most abundantly available red seaweed species, for example. Only a few cases of HMF production from agarose hydrolysate have been reported to date. Yang et al. obtained 40% 5-HMF yield from agarose using metal chloride in aqueous media,24 which is the most recognized study of 5-HMF production. In previous work by our group, 5-HMF and levulinic acid (LA) were produced as byproducts during the hydrolysis of agarose to produce galactose using acid and were subsequently recovered by nanofiltration and electrodialysis25 or by chromatography.26 Recently, acid hydrolysis of biomass materials has been increasingly done by using a solid acid catalyst instead of liquid acid, mainly because it can be recycled, saving material costs for the catalyst.17,18,27–34
In this study, 5-HMF was produced from agarose by using a solid acid catalyst, which can be recycled after the reaction to save material cost and at the same time eliminate the necessity of the subsequent neutralization step, which is mandatory when a liquid acid is used. The reaction was performed in a polar aprotic solvent instead of in aqueous phase to provide a more favorable micro-aqueous environment to 5-HMF formation. The effects of moisture content were investigated to identify the optimum level. The possibility of repeated use of the solid catalyst was also examined.
2. Experimental
2.1. Materials
Agarose (SeaKem® LE) was purchased from Lonza Group Ltd., Switzerland. 5-Hydroxymethylfurfural, levulinic acid, Amberlyst 36 (wet type), N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMA) and dimethyl sulfoxide (DMSO, anhydrous ≥99.9%) were purchased from Sigma-Aldrich Co. LLC. Formic acid was purchased from Junsei Chemical Co. Ltd., Japan.2.2. Reactors and reaction conditions
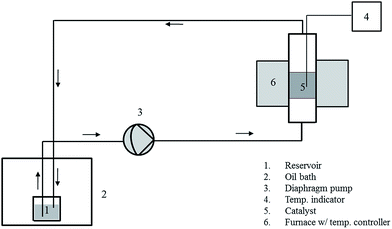
In beaker experiments, 5 g L−1 of agarose in 100 mL of DMF, DMA or DMSO was treated using 6 g (dry basis) of Amberlyst 36 at 140 °C for 180 min in a 250 mL beaker. The moisture content in the reaction mixture was varied in the range of 1.8–12.6% by adding the required amount of distilled water. When the reaction was performed in water for the purpose of comparison, an autoclave was used, due to the high vapor pressure of the reaction mixture, at 140 °C for 210 min. Experiments were also carried out in a packed-bed differential recycle reactor (Fig. 1). The total liquid volume was 300 mL, and 18 g of Amberlyst 36 was packed in the reactor. The reaction temperature was controlled at 140 ± 1.0 °C, and the reaction time was 180 min.2.3. Amberlyst 36 recycling
At the end of the reaction, Amberlyst 36 was taken out of the reactor and washed by using distilled water with vacuum filtering to remove the reaction products. The washed catalyst was exsiccated for 24 hours at 100 °C in an oven, and then recycled to the reactor for the next reaction run.2.4. Calculations
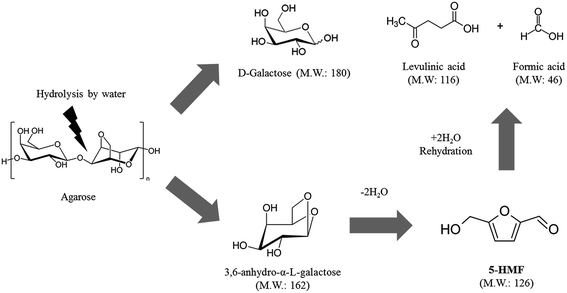
Agarose is composed of repeating dimeric galactosyl-anhydrogalactosyl (G-A) units.35 Its decomposition can be expressed as a 3 step-reaction: agarose hydrolysis to release galactose and 3,6-anhydro-α-L-galctose (AHG) (eqn (1)); 5-HMF formation from AHG by dehydration23,36 (eqn (2)); and rehydration of 5-HMF into LA and formic acid (FA) (eqn (3)) as depicted in Fig. 2.| C6H9O4–[C6H10O5 + C6H8O4]n−1–C6H11O6 + (2n − 1)H2O → nC6H12O6 + nC6H10O5 | (1) |
| C6H10O5 → C6H6O3 + 2H2O | (2) |
| C6H6O3 + 2H2O → C5H8O3 + CH2O2 | (3) |
The amount of agarose is presented in moles of dimeric G-A, and galactose yield from agarose is defined on a mole basis to be [M galactose/M G-A in the feed] as presented in eqn (4). The molar yields of 5-HMF, LA and FA are defined in the same way.
 | (4) |
2.5. Analytical procedures
5-HMF was quantified by using a high-performance liquid chromatograph (HPLC) (Dionex Inc., Korea) equipped with a Luna 5u C18 column (250 × 4.6 mm, Pheonomenex Inc., USA). Five% (v/v) of acetonitrile in distilled water was used as the mobile phase at a flow rate of 0.8 mL min−1, and the column temperature was 30 °C. Detection was done at a wavelength of 280 nm by using a UV detector (Dionex Inc., Korea).37 LA and FA were analyzed by using the same HPLC with an Aminex HPX-87H column (300 × 7.8 mm, Bio-Rad Laboratories, Inc., USA). Ten mM of H2SO4 was used at a flow rate of 0.6 mL min−1, and the column temperature was 65 °C. Its detection was done at a wavelength of 210 nm by using the UV detector mentioned earlier.38 The galactose concentration was measured by using another HPLC (Waters Corp., USA) with a Asahipak NH2P-50 4E column (250 × 4.6 mm, Shodex, Japan). 65% (v/v) of acetonitrile in distilled water was used as the mobile phase, at a flow rate of 1.0 mL min−1. The column temperature was 40 °C. An evaporative light scattering detector (Sedex 75, Sedere, France) was used.3. Results and discussion
3.1. Selection of solvent
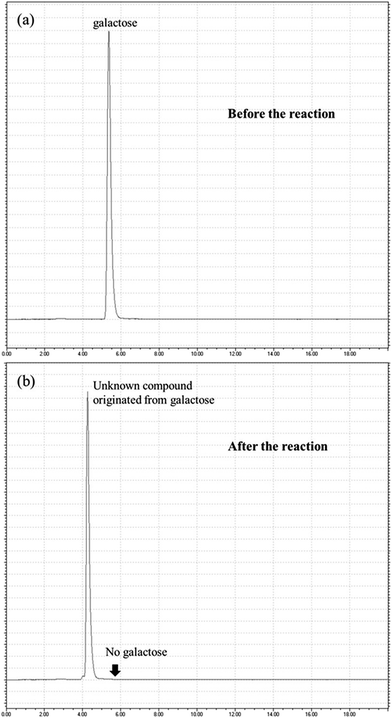
Agarose was treated with Amberlyst 36 in water and three different polar aprotic solvents: DMF, DMA and DMSO. When the reaction took place in water, 5-HMF yield increased in the beginning to reach rather a low value of 17% and then decreased as 5-HMF was rehydrated into LA and FA (Fig. 3a). The galactose yield was found to monotonically increase to reach 80%. On the contrary, 5-HMF yield monotonically increased while galactose yield showed a maximum when the polar aprotic solvents were used, as exemplified by the case with DMSO (Fig. 3b). The moisture content in DMSO was 5.4% (w/w). Among these three solvents, DMSO showed the highest 5-HMF yield of 50% (Table 1). For this reason, DMSO was chosen for the subsequent experimental study. The higher yield with DMSO might have been due to its own catalytic activity, as already reported.39 However, no noticeable amount of 5-HMF was formed when DMSO was used without Amberlyst 36 in this study (data not presented).One thing to note is that a much higher amount of galactose is produced in aqueous phase than in DMSO. In addition, no noticeable decrease in galactose yield with time is observed, while the decrease in galactose yield is significant after 120 min in DMSO. The reaction products in DMSO were analyzed by thin layer chromatography and HPLC (Fig. 4) to identify the cause for such loss of galactose. The chromatogram showed that the reaction mixture contains oligomeric compounds of neoagarotetraose and neoagarohexaose, galactose, and an unknown compound smaller than galactose. Considering that the two oligomers were from partial hydrolysis of agarose, it was speculated that the galactose formed from agarose hydrolysis was degraded into a low molecular weight compound. An experiment was performed with galactose in DMSO to prove such hypothesis. The results showed that as galactose disappeared, the same unknown compound was formed (Fig. 5). In a previous study by another group, galactose was found to be converted to glyceraldehyde, dihydroxyacetone or lactic acid.24 However, in this study, the unknown compound from galactose could not be identified.
3.2. Effects of moisture content
Theoretically speaking, an environment with no water is desirable for 5-HMF production, since it is formed through dehydration of AHG and then degraded into LA ad FA by rehydration. However, AHG is generated from agarose hydration, which is favored in an environment with high moisture content. It could be easily expected that the optimum moisture content for 5-HMF formation would exist, considering such contradictory effects of water. For the control of initial moisture content in the reaction mixture, Amberlyst 36, purchased in a wet form, was dried before being used for the reaction. It was desiccated for 24 hours in a furnace at 100 °C until no weight change was observed. The necessary amount of distilled water was added to adjust the moisture content at various levels from 1.8 to 12.6% (w/w). As expected, the 5-HMF yield increased as the moisture content increased to reach a maximum of 62% at 7.2% moisture content, and then decreased (Fig. 6). At this moisture content, LA yield was 11%. The FA yield is no longer presented, since it was identical to LA yield. | ||
| Fig. 6 5-HMF and levulinic acid yields at various levels of moisture content (5 g L−1 of agarose in distilled water, 60 g L−1 of Amberlyst 36, 140 °C, 180 min). | ||
It was notable that the LA yield was limited under a low level of 13% in DMSO regardless of moisture content in the range tested, while it was as high as 50% in aqueous solution. Mushrif et al. studied the molecular dynamics of solvent effects in the selective conversion of fructose to 5-HMF and provided a theoretical basis for the effects of DMSO solvation in protecting 5-HMF from rehydration.40 According to their theoretical and simulation work, DMSO solvates C1 and C2 carbons of 5-HMF and thus protects the bond between them from being cleaved during rehydration. Such a shielding effect of DMSO against 5-HMF rehydration well explains the limited LA yield observed in this study.
3.3. Recycling of Amberlyst 36
The main advantage of using a solid acid catalyst like Amberlyst 36 is its reusability, which can greatly contribute to the process economy, as discussed earlier in the Introduction section. For this reason, the number of repeated uses of Amberlyst 36 can be an important factor affecting process economy. A recycling test was performed by reusing the spent Amberlyst 36 after washing and desiccating for the two subsequent runs of experiment. Table 2 shows that the 5-HMF yield decreased from 62% in the fresh or first run to 55% in the third run, showing the feasibility of repeated use of Amberlyst 36 in the viewpoint of activity. It was found, however, that its repeated use would be not feasible due to its structural weakness. As shown in Fig. 7, Amberlyst 36 beads were almost completely broken down after three times of use due to attrition caused by mixing during the reaction. The loss in activity of Amberlyst 36 can be explained in two parts. The first is the detachment of sulfonic acid groups on the bead surface, and the second is the loss of powdery Amberlyst 36 during the recovery and washing steps between runs. Such structural fragility will be the main hurdle for Amberlyst 36 to be used in a mixed reactor, clearly suggesting the choice of other attrition-free reactors. In this study, a packed-bed differential recycle reactor was used to avoid the structural disintegration of Amberlyst 36. In a previous study by another group to produce 5-HMF from glucose by using the solid acid catalysts SO42−/ZrO2 and SO42−/ZrO2–Al2O3 in a magnetically stirred glass reactor, it was observed that the yield was about 34%, and SO42−/ZrO2–Al2O3 was found structurally robust and could be reused up to five times.27| 1st run | 2nd run | 3rd run | |
|---|---|---|---|
| 5-HMF yield (%) | 62 | 57 | 55 |
| LA yield (%) | 10 | 12 | 11 |
 | ||
| Fig. 7 Scanning electron microscope (SEM) images of Amberlyst 36: (a) before use (×100), (b) after the 3rd run (×100). | ||
3.4. 5-HMF production in a packed-bed reactor
As shown in Fig. 1, the reaction mixture was recycled through the catalyst packed in a glass tube reactor. In this situation, the recycle flow rate should be high enough to minimize or eliminate the external mass transfer problem, which can negatively affect the reactor performance. The 5-HMF yield showed practically no difference in the tested range of recycle flow rate of 300–1200 mL min−1 (Table 3). This meant 300 mL min−1 of recycle flow rate was high enough not to cause a mass transfer problem, and thus the recycle flow rate was fixed at 300 mL min−1 in subsequent experiments.| Flow rate (mL min−1) | ||||
|---|---|---|---|---|
| 300 | 600 | 900 | 1200 | |
| 5-HMF yield (%) | 53 ± 0.4 | 53 ± 0.2 | 51 ± 1.6 | 52 ± 0.3 |
Effects of the initial moisture content were investigated and compared with those from the beaker experiments (Fig. 8). The optimum moisture content was found to be 7.2% (w/w) again, consistent with the results in beaker. However, 5-HMF yield in the packed-bed reactor was 4–9% lower than that in beaker. Such results were rather unexpected, considering that a mixed-flow reactor like the beaker and a packed-bed reactor are, in principle, the same in terms of reaction performance except for the flow pattern inside the reactor. The higher catalytic activity in the beaker experiment might be explained, at least partially, by the breakage of catalyst beads due to attrition in this type of mixed-flow reactor, which offers an increased exposure of sulfonic acid groups to the reaction mixture.
 | ||
| Fig. 8 Comparison of 5-HMF yields in the beaker and packed-bed reactor (5 g L−1 of agarose in DMSO, 60 g L−1 of Amberlyst 36, 140 °C, 180 min). | ||
The main objective of employing a packed bed reactor is to protect the catalyst from the detrimental effects of attrition and thus to substantially improve its reusability or lifespan. To investigate the performance of Amberlyst 36 as it was repeatedly used, consecutive runs of experiments were carried out without changing the catalyst. After each run, the reaction mixture was completely drained from the reactor, and a fresh reaction mixture was added into the reactor for the next run. A sharp drop in 5-HMF yield was observed after the 1st run (Fig. 9). It decreased from 53% in the 1st run to about 34% after the 4th run. The abrupt drop after the 1st run was purely speculated to be due to the loss of sulfonic acid groups on the bead surface caused by the flow-induced shear force. The stabilized performance of Amberlyst 36 after the 4th run supports its good reusability, although no long-term reusability test has been done.
 | ||
| Fig. 9 Results of reusability testing in a packed-bed reactor (5 g L−1 of agarose in DMSO with 7.2% moisture content, 60 g L−1 of Amberlyst 36, 140 °C, 180 min). | ||
4. Conclusions
The production of 5-HMF through the hydrolysis of agarose, a renewable biomass material, is a promising alternative to chemical production from fossil resources such as petroleum and coal. It was demonstrated herein that DMSO, one of the polar aprotic solvents, played a role as an effective medium for 5-HMF production by providing a micro-aqueous environment. The moisture content in DMSO was found to be the governing factor for 5-HMF production, having an optimum level of 7.2% at which the 5-HMF yield was 62%. DMSO was also found to repress 5-HMF rehydration, which would lower its yield. It was proven that Amberlyst 36, the solid acid catalyst used in this study, could be repeatedly used in a packed-bed reactor, which was the prime objective for using a solid acid instead of a liquid acid as catalyst.Acknowledgements
This work was supported by the Advanced Biomass R&D Center (ABC) of the Global Frontier Project funded by the Ministry of Education, Science and Technology (ABC-2011-0031356).Notes and references
- J. N. Chheda, G. W. Huber and J. A. Dumesic, Angew. Chem., 2007, 46, 7164–7183 CAS.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493 RSC.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC.
- J. C. Serrano-Ruiz and J. A. Dumesic, in Catalysis for Alternative Energy Generation, Springer, 2012, DOI:10.1007/978-1-4614-0344-9_2.
- B. Kamm, Angew. Chem., Int. Ed. Engl., 2007, 46, 5056–5058 CrossRef CAS PubMed.
- M. Bicker, J. Hirth and H. Vogel, Green Chem., 2003, 5, 280–284 RSC.
- E. L. Kunkes, D. A. Simonetti, R. M. West, J. C. Serrano-Ruiz, C. A. Gärtner and J. A. Dumesic, Science, 2008, 322, 417–421 CrossRef CAS PubMed.
- J. Lewkowski, ARKIVOC, 2001, 17–54 Search PubMed.
- J. Lewkowski, Arch. Org. Chem., 2001, 17–54 Search PubMed.
- B. F. M. Kuster, Starch, 1990, 42, 314–321 CAS.
- S. Hu, Z. Zhang, J. Song, Y. Zhou and B. Han, Green Chem., 2009, 11, 1746 RSC.
- Y. Su, H. M. Brown, X. Huang, X.-d. Zhou, J. E. Amonette and Z. C. Zhang, Appl. Catal., A, 2009, 361, 117–122 CrossRef CAS PubMed.
- F. Jiang, Q. Zhu, D. Ma, X. Liu and X. Han, J. Mol. Catal. A: Chem., 2011, 334, 8–12 CrossRef CAS PubMed.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Cellulose, 2011, 18, 1327–1333 CrossRef CAS.
- N. Shi, Q. Liu, Q. Zhang, T. Wang and L. Ma, Green Chem., 2013, 15, 1967 RSC.
- S. Hu, Z. Zhang, Y. Zhou, J. Song, H. Fan and B. Han, Green Chem., 2009, 11, 873 RSC.
- F. Ilgen, D. Ott, D. Kralisch, C. Reil, A. Palmberger and B. König, Green Chem., 2009, 11, 1948 RSC.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr, Green Chem., 2010, 12, 1855 RSC.
- J. N. Chheda, Y. Roman-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342 RSC.
- S. Wang, Y. Du, P. Zhang, X. Cheng and Y. Qu, Korean J. Chem. Eng., 2014, 31(12), 2286–2290, DOI:10.1007/s11814-014-0143-y.
- T. Damartzis and A. Zabaniotou, Renewable Sustainable Energy Rev., 2011, 15, 366–378 CrossRef CAS PubMed.
- J.-H. Park, J.-J. Yoon, H.-D. Park, Y. J. Kim, D. J. Lim and S.-H. Kim, Int. J. Hydrogen Energy, 2011, 36, 13997–14003 CrossRef CAS PubMed.
- B. Kim, J. Jeong, S. Shin, D. Lee, S. Kim, H.-J. Yoon and J. K. Cho, ChemSusChem, 2010, 3, 1273–1275 CrossRef CAS PubMed.
- L. Yan, D. D. Laskar, S.-J. Lee and B. Yang, RSC Adv., 2013, 3, 24090 RSC.
- J. H. Kim, J. G. Na, J. W. Yang and Y. K. Chang, Bioresour. Technol., 2013, 140, 64–72 CrossRef CAS PubMed.
- E. Valentin, H.-G. Nam, P.-H. Kim, H. W. Joo, H. J. Shim, Y. K. Chang and S. Mun, Sep. Purif. Technol., 2014, 133, 297–302 CrossRef CAS PubMed.
- H. Yan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef CAS PubMed.
- Y. Yang, X. Xiang, D. Tong, C. Hu and M. M. Abu-Omar, Bioresour. Technol., 2012, 116, 302–306 CrossRef CAS PubMed.
- Y. Yu, X. Xi, L. Jia, Q. Wei-Yan, Y. Hong-Peng, L. Gui-Ying and H. Chang-Wei, Chem. Res. Chin. Univ., 2009, 25, 234–238 Search PubMed.
- X. Shen, Y. X. Wang, C. W. Hu, K. Qian, Z. Ji and M. Jin, ChemCatChem, 2012, 4, 2013–2019 CrossRef CAS PubMed.
- A. Takagaki, M. Ohara, S. Nishimura and K. Ebitani, Chem. Commun., 2009, 6276–6278, 10.1039/b914087e.
- A. J. Crisci, M. H. Tucker, J. A. Dumesic and S. L. Scott, Top. Catal., 2010, 53, 1185–1192 CrossRef CAS.
- K.-i. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10, 1849–1853 CrossRef CAS PubMed.
- M. Ohara, A. Takagaki, S. Nishimura and K. Ebitani, Appl. Catal., A, 2010, 383, 149–155 CrossRef CAS PubMed.
- K. t. Nijenhuis, in Thermoreversible Networks, Springer Berlin Heidelberg, 1997, vol. 130, ch. Agarose, pp. 194–202 Search PubMed.
- B. Yang, G. L. Yu, X. Zhao, G. L. Jiao, S. M. Ren and W. G. Chai, FEBS J., 2009, 276, 2125–2137 CrossRef CAS PubMed.
- Y. H. Li and X. Y. Lu, J. Zhejiang Univ., Sci., B, 2005, 6, 1015–1021 CrossRef PubMed.
- K. Lourvanij and G. L. Rorrer, Ind. Eng. Chem. Res., 1993, 32, 11–19 CrossRef CAS.
- R. M. Musau and R. M. Munavu, Biomass, 1987, 13, 67–74 CrossRef CAS.
- S. H. Mushrif, S. Caratzoulas and D. G. Vlachos, Phys. Chem. Chem. Phys., 2012, 14, 2637 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02911b |
| This journal is © The Royal Society of Chemistry 2015 |