A triphenylamine-based colorimetric and “turn-on” fluorescent probe for detection of cyanide anions in live cells†
Abstract
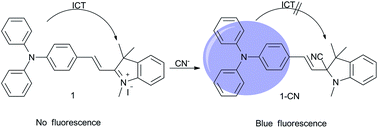
A colorimetric and “turn-on” fluorescent probe has been developed for the detection of cyanide anions. Cyanide was detected via the nucleophilic addition of cyanide to the indolium group of the probe, which resulted in a change from a purple colour to colourless and an enhancement in fluorescence. The probe showed a high sensitivity and selectivity for cyanide anions over other common anionic species in aqueous ethanol solution. The limit of detection was as low as 21 nM. A live cell imaging experiment demonstrated the practical value of this probe in tracing cyanide anions in biological systems.

 Please wait while we load your content...
Please wait while we load your content...