Silver nanoparticles: facile synthesis and their catalytic application for the degradation of dyes†
Abstract
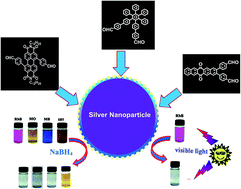
Derivatives 3, 5 and 7 having aldehyde groups at their periphery have been designed and synthesized. Interestingly, all three derivatives formed aggregates in aqueous media and served as reactors for facile synthesis of spherical silver nanoparticles (AgNPs) and silver nanorods at room temperature in aqueous media without the addition of any external reducing agents, stabilizing agents or shape directing additives. Interestingly, in situ generated AgNPs by aggregates of derivatives 3, 5 and 7 exhibit high catalytic degradation of industrially important organic dyes such as rhodamine B (RhB), methylene blue (MB), methyl orange (MO) and eriochrome black T (EBT).

 Please wait while we load your content...
Please wait while we load your content...