Significance of reagent addition sequence in the amidation of carboxylic acids mediated by PPh3 and I2†
Abstract
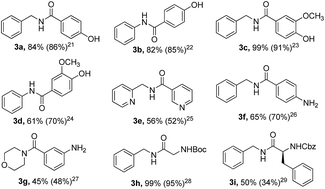
The outcome of the amidation reaction mediated by PPh3–I2 was found to be highly dependent on the addition sequence of the reagents. When triethylamine was subjected to a mixture containing PPh3, I2, and a carboxylic acid, acid anhydride was generated almost instantly, before treatment with an amine, presumably via an attack of carboxylate ion onto the acyl function of an acyloxyphosphonium salt. Nevertheless, when a PPh3–I2 mixture was treated with an amine, then a carboxylic acid, prior to adding the base, amide was rapidly formed in high yield with high chemoselectivity, most likely through an intermediate O,N-pentacoordinate phosphorane species as confirmed by ESI-MS technique.

 Please wait while we load your content...
Please wait while we load your content...