Robust reduced graphene oxide paper fabricated with a household non-stick frying pan: a large-area freestanding flexible substrate for supercapacitors†
Yang Huanga,
Minshen Zhua,
Wenjun Menga,
Yuqiao Fua,
Zifeng Wanga,
Yan Huanga,
Zengxia Peia and
Chunyi Zhi*ab
aDepartment of Physics and Materials Science, City University of Hong Kong, 83 Tat Chee Avenue, Kowloon, Hong Kong, P. R. China. E-mail: cy.zhi@cityu.edu.hk; Fax: +852-3442-0538; Tel: +852-3442-7891
bShenzhen Research Institute, City University of Hong Kong, Shenzhen, P. R. China
First published on 27th March 2015
Abstract
Inspired by cooking omelettes, a facile, low-cost and scalable method involving the use of a readily available household non-stick frying pan is introduced to fabricate large-area freestanding reduced graphene oxide (RGO) paper. The as-fabricated RGO paper is robust enough to bear sandpaper polishing, bending/folding, and hydrothermal and electrochemical deposition processes without obvious structure/performance degradation. As a demonstration, the as-obtained RGO papers were directly used as universal flexible substrates for high performance supercapacitors (SCs). Thus, WO3 and PPy, which are two distinctive active materials, were loaded onto the RGO paper via a hydrothermal process and electrodeposition process, respectively, which are two typical fabrication methods for high performance SC electrodes. The resultant WO3– and PPy–RGO paper, which act as negative and positive electrodes, respectively, were further assembled into a flexible asymmetric supercapacitor (ASC), achieving a high energy density of 0.23 mW h cm−3 at a power density of 7.3 mW cm−3 when normalized to the whole volume. Moreover, benefiting from the robust flexible RGO substrate, the performance of the ASC showed great stability under different bending angles and after repeated bending/folding. These exciting results demonstrate that our robust RGO paper is an ideal universal substrate for different active materials synthesized via various processing methods, which show its great potential in an all-solid energy storage system with excellent flexibility and robustness.
1. Introduction
The rapid rise of personal electronics, especially the ever-increasing demand for wearable electronics, has prompted the development of lightweight, flexible, and highly efficient energy storage devices.1,2 In accordance with this trend, a variety of flexible all-solid supercapacitors (SCs) combined with exceptionally long cycle life, enhanced high power and energy density, good operational safety and environmental benign nature have been designed and are currently being fabricated.3,4 For high performance flexible SCs, special attention has be dedicated to the design of flexible electrodes, and two main kinds of strategies have been developed, namely, (i) fabricating flexible hybrid electrodes of active materials5,6 and (ii) fabricating freestanding conductive substrates to support active materials.7,8 Compared with hybrid electrodes, the use of substrates is more attractive, not only because of their low cost and high flexibility, but also, more importantly, because they are more universal and their use could open up the possibility to support any upcoming active material with enhanced performance via different processing methods.Recently, metals,4 carbon-based materials,9 textiles,10 sponges11 and even conventional paper12,13 have been used as substrates to fabricate high performance flexible SCs. Among these, graphene-based materials, one of the most commonly reported carbon materials lately, have attracted significant attention owing to their outstanding electronic, mechanical, and thermal properties,14,15 which can lead to a wide range of applications such as conducting transparent electrodes,16 electrochemical batteries,17 sensors,18 and SCs.19 Because of their 2-dimensional properties, these graphene-based materials can be easily assembled into macroscopic paper-like structures, making them an ideal flexible substrate for loading active materials. Generally, there are two main approaches for obtaining graphene-based paper: one is by direct production using reduced graphene oxide (RGO),6,20 and the other is to rely on the post-reduction of pre-fabricated GO paper.21 However, current strategies for the production of freestanding RGO paper substrates are far from satisfactory, since they are usually time consuming (e.g. membrane filtration)6 and/or require custom accessories or delicate equipment (e.g. electrophoretic deposition and chemical vapor deposition (CVD)),22,23 which may impede their further development in large-scale fabrication applications. Obviously, the most important obstacle is to achieve a large-area RGO paper via a simple method with high efficiency.
Herein, we develop an ingenious method to fabricate a large-area freestanding RGO paper by using common household utensils, which is demonstrated to be a facile, low cost and scalable method that was inspired by cooking an omelette. After successful post-reduction, the obtained RGO paper exhibits excellent mechanical and electrical properties, as well as good compatibilities with many different active materials and processing methods, which enable it to be directly used as a robust substrate for high performance SCs. As a demonstration, two distinctive active materials (WO3 and PPy) were loaded on to an RGO substrate perfectly via hydrothermal and electrodeposition methods, which are the two typical synthesis methods for SC electrodes. These two resultant flexible electrodes were further assembled into high performance all-solid asymmetric supercapacitors (ASCs) and were able to achieve a high energy density of 0.23 mW h cm−3 at a power density of 7.3 mW cm−3. Moreover, these ASCs showed no degradation under different bending angles and remained stable even after hundreds of times of being bent, together with excellent cycling stability (retaining 99.4% after 2000 cycles). Therefore, the RGO paper, with excellent flexibility and robustness, is proven to be an ideal universal substrate for various active materials synthesized via different processing methods, thus showing great potential for various portable/wearable electronics.
2. Experimental section
2.1 Fabrication of the RGO paper
GO was prepared from purified natural graphite by using a modified Hummers method.24 A suspension of GO sheets was obtained by ultrasonic exfoliation of the as-prepared GO in deionized (DI) water, followed by centrifugation at 5000 rpm for 10 min to remove the non-exfoliated sheets.Our ingenious fabrication of a large area freestanding RGO paper was inspired by the daily cooking of an omelette, as shown in the schematic in Fig. 1A. First, an adequate amount of GO suspension (5 mg mL−1, 100 mL), a viscous liquid similar to egg liquid, was poured directly into a common household non-stick frying pan (IKEA, with a diameter of 20 cm) using a spatula to ensure even spreading. Then, the frying pan was placed on an induction cooker and kept under appropriate heating conditions (60 W, 80–90 °C) until the GO suspension was dried, normally requiring 2–3 hours. It is suggested that the heating temperature is maintained under 100 °C, since small pores will form if the suspension solution boils. Then, the GO paper was easily peeled off form the frying pan and reduced into RGO paper by immersing into a hydrohalic acid (HI) solution in a sealed cuvette.21 Finally, the large area freestanding RGO paper was annealed at 750 °C under an argon flow of 50 mL min−1 for 2 h, which was employed for elimination of the introduced sub-products (I− ions), while also providing extra reduction.
2.2 Fabrication of WO3– and PPy–RGO paper
The RGO paper was first cut into an appropriate size (e.g. 5 cm × 2 cm) and polished with sandpaper (P1000) in order to obtain a unique fish scale-like structure. The pre-treated paper was washed with DI water and dried prior to use.The WO3–RGO paper electrode was prepared by a hydrothermal method, using RGO paper as the substrate according to a previous report.25 10 mL of 0.3 M HCl was slowly added into the 10 mL solution containing 0.6 g Na2WO4·2H2O, and then 0.6 g NaCl was added into the solution. Subsequently, the RGO paper was immersed in the resultant solution and transferred into a Teflon-lined stainless autoclave, and then heated at 180 °C for 12 h. After the autoclave cooled down to room temperature, the RGO paper was taken out, rinsed with DI water and dried prior to use. The precipitates were collected by centrifugation (5000 rpm), washed with DI water and absolute ethanol several times, and then finally dried overnight.
The PPy–RGO paper electrode was prepared by an electrodeposition method using a three-electrode configuration with RGO paper as the working electrode, platinum mesh as the counter electrode, and Ag/AgCl as the reference electrode. In brief, the electrodeposition solution contained 300 μL distilled pyrrole monomer, 0.1 M p-toluenesulfonic acid, 0.3 M sodium dodecylbenzenesulfonate and 60 mL DI water. A constant voltage of 0.8 V was used during the deposition process and lasted for 90 s. Then the PPy–RGO paper was washed with DI water and dried prior to use.
2.3 Assembly of WO3–RGO//PPy–RGO ASC
The WO3– and PPy–RGO papers, acting as the negative and positive electrodes, were assembled into an ASC by using PVA–H2SO4 as the solid electrolyte. The PVA–H2SO4 gel electrolyte was first prepared by dissolving PVA powder (6 g) in DI water (60 mL) and H2SO4 (6 g). The mixture was heated to 150 °C with vigorous stirring until a transparent solution formed. Then, the RGO-based electrodes were coated with a thin layer of PVA–H2SO4 gel electrolyte and dried under vacuum at room temperature. Afterwards, another thin layer of gel electrolyte was coated on one of the resultant electrodes, by pasting the other onto it gently and then drying in air. Thus, a flexible all-solid ASC was obtained.2.4 Materials characterization and electrochemical measurements
The morphology of the samples was investigated with an environmental scanning electron microscope (ESEM, FEI/Philips XL30). X-ray diffraction (XRD) patterns were collected with a BRUKER D2 PHASER diffractometer equipped with CuKα irradiation (λ = 1.54184 Å, 30 kV and 10 mA). X-ray photoelectron spectroscopy (XPS) measurements were performed on an ESCALAB 220i-XL electron spectrometer with monochromatic Al Kα X-ray radiation and a hemispherical electron energy analyzer. Raman spectra were excited with a laser of 523 nm and recorded with a Labram spectrometer. The electrical conductivity of the RGO paper was measured by a standard four-probe method. Mechanical property measurements were performed on strips of the papers with an Instron 5661 tester (tensile rate = 0.020 mm min−1). Sample widths were measured by a vernier caliper, while the thicknesses were measured on the fracture edges with SEM. Electrochemical measurements, including cyclic voltammetry (CV) curves, galvanostatic charge–discharge curves, and electrochemical impedance spectroscopy (EIS, 100 kHz–0.05 Hz) were conducted on a CHI 760e electrochemical workstation (CH Instruments Company). The electrochemical performances of the individual electrodes were investigated using a conventional three electrode system (with Pt mesh as the counter electrode and Ag/AgCl as the reference electrode) in 1 M H2SO4 prior to assembling of the ASC. The performance of the ASC was measured using a two-electrode method.2.5 Calculations
The areal (Cs, F cm−2) and specific (Cm, F g−1) capacitance of the positive and negative electrodes in the three electrode configuration were calculated from the corresponding charge–discharge curves at different current densities according to eqn (1):| Cs = IΔt/sΔV or Cm = IΔt/mΔV | (1) |
The capacitance values of the ASC were calculated from the corresponding CV curves at different scan rates according to eqn (2):
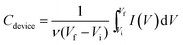 | (2) |
| Cs = Cdevice/A or CV = Cdevice/V | (3) |
The volumetric energy density of the ASC was obtained from the following equation:
 | (4) |
The power density of the device was calculated from the following equation:
 | (5) |
3. Results and discussion
3.1 Fabrication of large area freestanding RGO paper
As shown in Fig. 1A, when the water keeps evaporating under continuous heating and leads to a higher and higher GO concentration, the supersaturated GO sheets floating on the surface will aggregate together and self-stack into a thin film because of the increasing sheet-to-sheet interaction, thus ultimately forming a layer-by-layer GO paper. Because of the Teflon-lined coating, the dried dark brown round-shaped GO paper can be easily peeled off from the frying pan (Fig. S1†). Then, this GO paper can be easily reduced into a highly conductive RGO paper with HI solution and by thermal annealing. Based on this ingenious method, large-area freestanding flexible RGO papers with a variety of sizes and thicknesses can be obtained by just changing to non-stick pans of different sizes or by simply adjusting the volume and concentration of the GO suspension. As a demonstration, a GO paper with a surface area of 144 cm2 was made and could be folded into an origami crane. After unfolding, amazingly, there were only several creases left on the paper. (Fig. 1B) Then, this pristine GO paper was reduced into RGO paper by the aforementioned methods. The RGO paper demonstrated similar flexibility and toughness with the pristine GO paper, showing no defects even after complex folding (Fig. 1C). When compared to other reported fabrication methods for RGO paper,22,26–30 our ingenious approach has many advantages: (i) it is convenient and easy to scale up as the equipment is a common household non-stick frying pan, which is readily available; (ii) there is a possibility to fabricate RGO papers with multiple sizes; (iii) it is efficient and evaporation of water from the GO suspension exhibits a satisfactory efficiency, especially compared with the traditional time-consuming, vacuum-assisted flow-filtration methods; and (iv) it is cost-effective since the frying pan can be readily reused after cleaning, and thus the cost is extremely low.3.2 Characterization
The deoxygenation and interlaminar consolidation of the as-prepared papers was proven by the detailed characterization of XRD and XPS. As shown in Fig. 2A, the XRD pattern of the GO paper presents a diffraction peak at 2θ = 9.38°, suggesting a larger interlayer spacing (0.94 nm) due to the presence of oxygen-containing functional groups (e.g. epoxy and hydroxyl groups) attached to the GO sheet surface and molecules of water coming from the harsh oxidation applied.31 In the case of RGO paper, the diffraction peak of RGO is shifted to 25.64°, becoming narrower and sharper, not only indicating a significant reduction of the interlayer distance (0.35 nm) owing to the elimination of oxygen-containing groups, but also revealing the more regular packing of graphene layers with a longer correlation length due to the coalescence of the graphene sheets.21 The high resolution of the C1s peaks in the XPS of the RGO paper also proves that the oxygen-containing groups have been removed (Fig. 2B). Deconvolution of the C1s spectrum in the pristine GO paper reveals that it consists of two main components arising from C–O (hydroxyl and epoxy, ∼286.3 eV) and C–O–C (∼286.9 eV) and one minor component C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/O–C
O/O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl, ∼288.3 eV). After the post-reductions, it is clear that the majority of the oxygen-containing groups in the pristine GO paper are almost all removed and the C–C/C
O (carbonyl, ∼288.3 eV). After the post-reductions, it is clear that the majority of the oxygen-containing groups in the pristine GO paper are almost all removed and the C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds then become dominant (∼284.7 eV) in the RGO paper, as shown by the one single peak with a small tail in the higher binding energy region in Fig. 2B. The surface C/O atomic ratio detected by XPS is ∼2.8 for the pristine GO paper and is increased to ∼12.5 after the post-reductions, strongly indicating the complete elimination of the oxygen groups.
C bonds then become dominant (∼284.7 eV) in the RGO paper, as shown by the one single peak with a small tail in the higher binding energy region in Fig. 2B. The surface C/O atomic ratio detected by XPS is ∼2.8 for the pristine GO paper and is increased to ∼12.5 after the post-reductions, strongly indicating the complete elimination of the oxygen groups.
The removal of the oxygen-containing groups leads to greater connectivity among the existing graphitic domains by the formation of new sp2 clusters,32 which can also be followed by Raman spectroscopy. The G-band (at 1585 cm−1) is known to be a characteristic of the sp2-hybridized carbon–carbon bonds in graphene.33,34 Moreover, the presence of the D-band (at 1350 cm−1) indicates structural imperfections induced by the attachment of oxygen-containing functional groups on the carbon basal plane.34 As a result, the ID/IG ratio is widely used to provide structural information on the GO with different degrees of reduction, while a high ID/IG is related to the presence of disordered carbon and/or functionalities attached to graphene sheets. Compared with GO paper, the intensity of the D-band peak in RGO paper is clearly reduced and the ID/IG ratio is decreased notably from 1.31 to 0.82, suggesting the extensive repairing of defects created by the attachment of oxygen groups.
Owing to restoration of the sp2 carbon network in the GO sheets and the decrease in the interlayer distance, as revealed in the structural characterization, the interaction among the reduced GO sheets in RGO paper is increased; thus, the electrical conductivity and mechanical strength of the RGO paper are greatly improved. The conductivities of the RGO paper were measured by a collinear four-point probe method, and a high value for electrical conductivity was achieved by our progressive reduction, reaching as high as 203.7 S cm−1. Due to the increased neighbouring coalescence interactions between the RGO sheets, the RGO paper has a modulus of 1.65 GPa and a strength of 9.41 MPa, which is 11 times and 21 times more than that of the pristine GO paper (modulus 0.13 GPa, strength 0.41 MPa) (Fig. 3A). Accordingly, RGO paper presents outstanding flexibility and toughness, as shown in Fig. 1C. To further investigate whether the good electrical conductivity of the RGO paper is in agreement with its robust body, cycled bending tests of the resistance were operated by a designed stepper motor. It is very impressive that the resistance showed no observable change after repeated bending for over 1000 times (Fig. 3B), indicating that the good electrical conductivity was integrated with its robust body. Good electrical conductivity, together with excellent mechanical strength, is important for applying the flexible substrates to high-performance SC electrodes, as they can ensure that the substrate can bear various post-treatments and fabrication processes.35 Based on the above demonstrations, our RGO paper should be an ideal substrate for SCs.
3.3 Universal robust substrate for a high-performance SC electrode
To further investigate the robust RGO paper as a universal substrate for different active materials synthesized via different processing methods, two different kinds of electrodes with well-designed structures were fabricated by a hydrothermal (WO3) method and an electrodeposition (PPy) method, respectively, which are two representative processing methods for high-performance SCs4,36 (Fig. 4A). One large free-standing flexible RGO paper is firstly cut into the appropriate size (e.g. 5 cm × 2 cm, 10 cm2), and then polished with sandpaper along one direction; hence, the smooth surface of RGO paper becomes rougher and exhibits a unique fish scale-like structure, as shown in Fig. 4B. These micro fish scale-like RGO sheets, which are torn off from the bulk self-stacked RGO paper, ensure that more active materials could be loaded on to the substrate, which may result in a better electrochemical performance. Benefiting from the robust features, the polished RGO paper can bear both the hydrothermal and electrodeposition methods, turning the conductive substrate into a flexible electrode. As shown in Fig. 4C, after hydrothermal treatment, numerous nanoparticles, which were characterized to be WO3 (JCPDS no. 75-2187, Fig. S2†), are anchored both on the rough surface and on the fish scale-like sheets; however, after electrodeposition, the scratches and steps were clearly alleviated, since a thin layer of PPy, which is a typical conductive polymer, was successfully coated on the rough surface.Due to their excellent conductivity and integrated structure, the free-standing WO3-loaded and PPy-coated RGO papers can be directly used as SC electrodes. A three-electrode test configuration was employed to evaluate the electrochemical performance of both WO3– and PPy–RGO paper electrodes. Fig. 5A and C show the CV loops of both electrodes in the range of 10–100 mV s−1. The CV curve of the WO3–RGO electrode deviates from a rectangular shape, which can be attributed to the relatively large resistance, while the CV curve of PPy–RGO electrode is rectangular at the applied scan rates, indicating an excellent capacitive behaviour and a low resistance.37 The charge and discharge curves at different charge currents are shown in Fig. 5B and D. The deviation of the charge–discharge curves from the linear voltage–time relation of the WO3–RGO electrode indicates a pseudo-capacitive behaviour (caused by the redox reactions),38 whereas the curves of the PPy–RGO electrode are highly linear and symmetrical, suggesting a very good capacitive behaviour.39 Moreover, no obvious iR drop is observed for any of the curves, indicating a rapid I–V response and an excellent electrochemical reversibility.6 The specific capacitance is calculated to be 123.4 F g−1 (WO3–RGO) and 127.5 F g−1 (PPy–RGO) at a current density of 1 A g−1, corresponding to area capacitance of 71 mF cm−2 and 80 mF cm−2.
3.4 Assembly of a flexible all-solid state ASC
The robust freestanding RGO paper, which is compatible with distinctive active materials and can perfectly bear various processing methods, is an ideal substrate for flexible SC electrodes. To further demonstrate its promising application in high performance power devices with excellent flexibility, an all-solid state ASC was further fabricated by assembling the as-prepared WO3– and PPy–RGO papers as negative and positive electrodes, respectively. The mass ratio of the two electrodes was fixed to 1.24, in order to achieve a charge balance between the negative and positive electrodes (Fig. 6A).The stable operating potential windows for the WO3– and PPy–RGO electrodes are −0.5 to 0 V and 0 to 0.6 V, respectively (Fig. 6B). The cell voltage can be expressed as the sum of the potential range of the electrodes, and consequently the asymmetric device is expected to work within a voltage window of −0.5 to 0.6 V. A series of CV measurements under different voltage windows are shown in Fig. 6C. The device exhibits a stable potential window even up to 1.3 V, which exceeds the theoretical decomposition voltage of water (1.23 V) and could be ascribed to the high overpotential of hydrogen evolution on the electrode.40
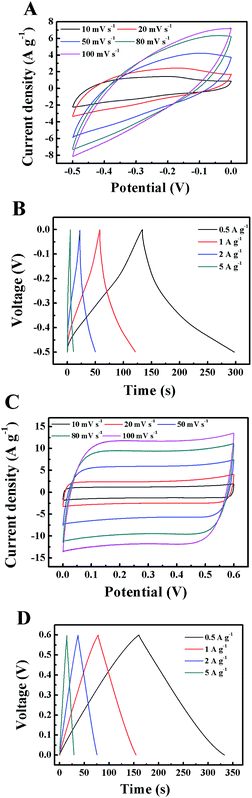
CV curves at different scan rates (ranging from 10 to 200 mV s−1) are collected in order to evaluate the performances of the WO3–RGO//PPy–RGO ASC, as shown in Fig. 7A. The assembled full cells exhibit quasi-rectangular CV curves at low scan rates, which is attributed to the combination of the non-rectangular curves of WO3 and the rectangular curves of PPy, which agrees with a good capacitive behaviour.41 Moreover, the CV curve still remains symmetrical even at a high scan rate of 400 mV s−1 (Fig. S3†), indicating good reversibility of the electrochemical processes.40 The charge–discharge curves show a symmetric charge and discharge process, and the deviation from the linear curve is attributed to the pseudo-capacitive behaviour of WO3–RGO. The volumetric stack capacitance of the ASC was calculated to be 1.31 F cm−3 at a scan rate of 10 mV s−1, which is much larger than the values reported for graphene (0.42 F cm−3),42 TiN (0.33 F cm−3)43 SC and H–TiO2@MnO2//H–TiO2@C (0.7 F cm−3)44 all-solid ASC. Correspondingly, the area capacitance was 65.7 mF cm−2, which was higher than the graphene–cellulose-paper-based SC (47 mF cm−2).20 Note that compared with many other reported graphene-based planar films or papers,6,20,42 our RGO paper is merely acting as a highly conductive flexible substrate and its contribution to the capacitance is very limited (Fig. S4†), which can eliminate the unnecessary distractions of capacitive performance and provide an accurate evaluation of the loaded active materials.
As per previous demonstrations, the RGO substrate exhibits outstanding flexibility and toughness. Thus, it is predictable that the ASC based on the RGO electrodes has great potential as a flexible power source. As shown in Fig. 7C, the perfectly overlapped CV curves at different bending angles indicate that the ASC can be bent to a large extent without degrading the performance. Moreover, even after being folded and unfolded to 180° over 200 times, the CV curves do not show an obvious change (Fig. S5†). A Ragone plot, which shows the energy and power densities (normalized to the whole device volume), is given in Fig. 7D. Significantly, our ASC possesses a high volumetric energy density of 0.23 mW h cm−3 at a power density of 7.3 mW cm−3, which is comparable with or better than those of the previously reported flexible ASC or SCs such as hydrogenated TiO2@MnO2//TiO2@C-based ASC (0.30 mW h cm−3),44 MnO2/carbon particles-based SC (0.09 mW h cm−3),45 and graphene-based SC (0.06 mW h cm−3).42 Moreover, our device shows remarkable cycling stability with a high capacitance retention of 99.4% over 2000 cycles at 8.3 mA cm−3, which is significant for practical applications. This outstanding cycling performance is highly competitive with those of other reported ASCs such as Co(OH)2/activated carbon (93% retention after 1000 cycles),38 graphene/MnO2/activated carbon nanofiber (97% retention after 1000 cycles),6 MnO2/activated carbon (96% retention after 1000 cycles),46 and MnO2/functional mesoporous carbon nanotubes (90% retention after 1000 cycles).47 EIS was used to investigate the resistance change of the ASC before and after the cycling test. After 2000 cycles, nearly no change was observed for Rct (Ω), and only a slight increase of Rs from 36 to 50 Ω was observed. These EIS results further demonstrate the exceptional stability of our flexible ASC, which is attributed to the robust RGO substrate.
4. Conclusions
In summary, inspired by cooking an omelette, a large area freestanding pristine GO paper was firstly fried using a non-stick frying pan. This was followed by post-reductions, when the GO paper was reduced to RGO paper with excellent electrical and mechanical properties, as well as outstanding flexibility and robustness. In particular, this ingenious method can fabricate RGO papers in a variety of sizes and thicknesses just by simply changing the frying pan or the concentration of the GO solution. The as-fabricated RGO papers are very robust, being able to bear sandpaper polishing, bending, folding, and hydrothermal and electrochemical deposition processes without any structure/performance degradation. As a demonstration, two flexible RGO-based electrodes were fabricated via hydrothermal and electrodeposition methods with WO3 and PPy as the active materials, respectively. The as-obtained WO3– and PPy–RGO electrodes were further assembled into an all-solid ASC, which achieved a high energy density of 0.23 mW h cm−3 at a power density of 7.3 mW cm−3 and an outstanding retention rate of 99.4% after 2000 cycles. In addition, benefiting from the robust features of RGO paper, the flexible all-solid ASC possesses a great deformation tolerance. Consequently, the robust RGO paper, as developed by our facile, low-cost and scalable method, can be applied to fabricate high performance flexible power sources, thus showing great potential for portable wearable electronic devices.Acknowledgements
This research was supported by the Early Career Scheme of the Research Grants Council of Hong Kong SAR, China (under Project Numbers City U 9041977), the Science Technology and Innovation Committee of Shenzhen Municipality (Grant number R-IND4901), and a grant from the City University of Hong Kong.Notes and references
- Z. Liu, J. Xu, D. Chen and G. Shen, Chem. Soc. Rev., 2015, 44, 161–192 RSC.
- G. Shen, L. Liao, C. Zhou and Y. Bando, J. Mater. Chem. C, 2014, 2, 1176–1177 RSC.
- Z. Zhang, J. Deng, X. Li, Z. Yang, S. He, X. Chen, G. Guan, J. Ren and H. Peng, Adv. Mater., 2015, 27, 356–362 CrossRef CAS PubMed.
- Y. Huang, J. Tao, W. Meng, M. Zhu, Y. Huang, Y. Fu, Y. Gao and C. Zhi, Nano Energy, 2015, 11, 518–525 CrossRef CAS PubMed.
- W. Tian, X. Wang, C. Zhi, T. Zhai, D. Liu, C. Zhang, D. Golberg and Y. Bando, Nano Energy, 2013, 2, 754–763 CrossRef CAS PubMed.
- Z. Fan, J. Yan, T. Wei, L. Zhi, G. Ning, T. Li and F. Wei, Adv. Funct. Mater., 2011, 21, 2366–2375 CrossRef CAS PubMed.
- J. Tao, N. Liu, L. Li, J. Su and Y. Gao, Nanoscale, 2014, 6, 2922–2928 RSC.
- S. Wang, N. Liu, J. Tao, C. Yang, W. Liu, Y. Shi, Y. Wang, J. Su, L. Li and Y. Gao, J. Mater. Chem. A, 2015, 3, 2407–2413 CAS.
- R. Ma, X. Liu, J. Liang, Y. Bando and T. Sasaki, Adv. Mater., 2014, 26, 4173–4178 CrossRef CAS PubMed.
- X. Lu, T. Zhai, X. Zhang, Y. Shen, L. Yuan, B. Hu, L. Gong, J. Chen, Y. Gao, J. Zhou, Y. Tong and Z. L. Wang, Adv. Mater., 2012, 24, 938–944 CrossRef CAS PubMed.
- P. Li, Y. Yang, E. Shi, Q. Shen, Y. Shang, S. Wu, J. Wei, K. Wang, H. Zhu, Q. Yuan, A. Cao and D. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 5228–5234 CAS.
- B. Yao, L. Yuan, X. Xiao, J. Zhang, Y. Qi, J. Zhou, J. Zhou, B. Hu and W. Chen, Nano Energy, 2013, 2, 1071–1078 CrossRef CAS PubMed.
- L. Yuan, X. Xiao, T. Ding, J. Zhong, X. Zhang, Y. Shen, B. Hu, Y. Huang, J. Zhou and Z. L. Wang, Angew. Chem., Int. Ed., 2012, 124, 5018–5022 CrossRef PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed.
- A. L. M. Reddy, A. Srivastava, S. R. Gowda, H. Gullapalli, M. Dubey and P. M. Ajayan, ACS Nano, 2010, 4, 6337–6342 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2009, 110, 132–145 CrossRef PubMed.
- X. Wang, Y. Zhang, C. Zhi, X. Wang, D. Tang, Y. Xu, Q. Weng, X. Jiang, M. Mitome, D. Golberg and Y. Bando, Nat. Commun., 2013, 4, 1–8 CrossRef CAS.
- Z. Weng, Y. Su, D.-W. Wang, F. Li, J. Du and H.-M. Cheng, Adv. Energy Mater., 2011, 1, 917–922 CrossRef CAS PubMed.
- S. Pei, J. Zhao, J. Du, W. Ren and H.-M. Cheng, Carbon, 2010, 48, 4466–4474 CrossRef CAS PubMed.
- M. Wang, L. D. Duong, J.-S. Oh, N. T. Mai, S. Kim, S. Hong, T. Hwang, Y. Lee and J.-D. Nam, ACS Appl. Mater. Interfaces, 2014, 6, 1747–1753 CAS.
- K. Rana, S. D. Kim and J.-H. Ahn, Nanoscale, 2015 10.1039/c4nr06082b.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- M. Zhu, W. Meng, Y. Huang, Y. Huang and C. Zhi, ACS Appl. Mater. Interfaces, 2014, 6, 18901–18910 CAS.
- B. Shen, W. Zhai and W. Zheng, Adv. Funct. Mater., 2014, 24, 4542–4548 CrossRef CAS PubMed.
- G. Xin, H. Sun, T. Hu, H. R. Fard, X. Sun, N. Koratkar, T. Borca-Tasciuc and J. Lian, Adv. Mater., 2014, 26, 4521–4526 CrossRef CAS PubMed.
- C. Chen, Q.-H. Yang, Y. Yang, W. Lv, Y. Wen, P.-X. Hou, M. Wang and H.-M. Cheng, Adv. Mater., 2009, 21, 3007–3011 CrossRef CAS PubMed.
- D.-W. Wang, F. Li, J. Zhao, W. Ren, Z.-G. Chen, J. Tan, Z.-S. Wu, I. Gentle, G. Q. Lu and H.-M. Cheng, ACS Nano, 2009, 3, 1745–1752 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270–274 CrossRef CAS PubMed.
- J. Che, L. Shen and Y. Xiao, J. Mater. Chem., 2010, 20, 1722–1727 RSC.
- C. Mattevi, G. Eda, S. Agnoli, S. Miller, K. A. Mkhoyan, O. Celik, D. Mastrogiovanni, G. Granozzi, E. Garfunkel and M. Chhowalla, Adv. Funct. Mater., 2009, 19, 2577–2583 CrossRef CAS PubMed.
- A. Gupta, G. Chen, P. Joshi, S. Tadigadapa and P. C. Eklund, Nano Lett., 2006, 6, 2667–2673 CrossRef CAS PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS.
- D. P. Dubal, J. G. Kim, Y. Kim, R. Holze, C. D. Lokhande and W. B. Kim, Energy Technol., 2014, 2, 325–341 CrossRef PubMed.
- W. Meng, W. Chen, L. Zhao, Y. Huang, M. Zhu, Y. Huang, Y. Fu, F. Geng, J. Yu, X. Chen and C. Zhi, Nano Energy, 2014, 8, 133–140 CrossRef CAS PubMed.
- L. Hu, W. Chen, X. Xie, N. Liu, Y. Yang, H. Wu, Y. Yao, M. Pasta, H. N. Alshareef and Y. Cui, ACS Nano, 2011, 5, 8904–8913 CrossRef CAS PubMed.
- L.-B. Kong, M. Liu, J.-W. Lang, Y.-C. Luo and L. Kang, J. Electrochem. Soc., 2009, 156, A1000–A1004 CrossRef CAS PubMed.
- R. Liu and S. B. Lee, J. Am. Chem. Soc., 2008, 130, 2942–2943 CrossRef CAS PubMed.
- J. Liu, L. Zhang, H. B. Wu, J. Lin, Z. Shen and X. W. Lou, Energy Environ. Sci., 2014, 7, 3709–3719 CAS.
- T. Cottineau, M. Toupin, T. Delahaye, T. Brousse and D. Bélanger, Appl. Phys. A, 2006, 82, 599–606 CrossRef CAS PubMed.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS PubMed.
- X. Lu, G. Wang, T. Zhai, M. Yu, S. Xie, Y. Ling, C. Liang, Y. Tong and Y. Li, Nano Lett., 2012, 12, 5376–5381 CrossRef CAS PubMed.
- X. Lu, M. Yu, G. Wang, T. Zhai, S. Xie, Y. Ling, Y. Tong and Y. Li, Adv. Mater., 2013, 25, 267–272 CrossRef CAS PubMed.
- L. Yuan, X.-H. Lu, X. Xiao, T. Zhai, J. Dai, F. Zhang, B. Hu, X. Wang, L. Gong, J. Chen, C. Hu, Y. Tong, J. Zhou and Z. L. Wang, ACS Nano, 2011, 6, 656–661 CrossRef PubMed.
- H.-Q. Wang, Z.-S. Li, Y.-G. Huang, Q.-Y. Li and X.-Y. Wang, J. Mater. Chem., 2010, 20, 3883–3889 RSC.
- H. Jiang, C. Li, T. Sun and J. Ma, Nanoscale, 2012, 4, 807–812 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02868j |
| This journal is © The Royal Society of Chemistry 2015 |