Nb-doped VOx/CeO2 catalyst for NH3-SCR of NOx at low temperatures†
Zhihua Lian,
Fudong Liu‡
*,
Hong He* and
Kuo Liu
State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, PR China. E-mail: fudongliu@lbl.gov; lfd1982@gmail.com; honghe@rcees.ac.cn; Fax: +86 10 62849123; Tel: +86 10 62849123
First published on 20th April 2015
Abstract
The promotion effect of Nb addition to VOx/CeO2 catalysts for the selective catalytic reduction of NOx by NH3 was fully investigated. VOx/CeO2 and NbOx doped VOx/CeO2 catalysts were characterized by N2 physisorption, XRD, H2-TPR and NH3-TPD. The results showed that the addition of Nb could significantly promote the SCR activity of the VOx/CeO2 catalyst, especially in the low temperature range. VOx/CeO2 with 30 wt% NbOx catalyst showed the best catalytic performance and better SO2/H2O tolerance than VOx/CeO2 catalyst. 30Nb-1VOx/CeO2 also exhibited higher NH3-SCR activity than 3V2O5–WO3/TiO2. Lower crystallinity, stronger redox capability and more Brønsted acid sites of the Nb-VOx/CeO2 catalyst were all responsible for its more excellent NH3-SCR performance. Based on kinetic experiments and in situ DRIFTS results, it was concluded that the Langmuir–Hinshelwood mechanism existed for selective catalytic reduction of NO over Nb-VOx/CeO2, in which adsorbed NOx species reacted with adsorbed NH3 to finally form N2 and H2O.
1 Introduction
Nitrogen oxides (NO and NO2), which result from automobile exhaust gas and industrial combustion of fossil fuels, are major air pollutants.1 They contribute to a variety of environmentally harmful effects such as photochemical smog, acid rain, and haze formation.2 The selective catalytic reduction of NOx with NH3 (NH3-SCR) in the presence of excess oxygen is now the most efficient technology for the removal of nitrogen oxides from stationary sources.2,3 V2O5–WO3(MoO3)/TiO2 has been widely applied as an industrial catalyst for several decades.4,5 However, some problems still remain for V2O5–WO3(MoO3)/TiO2, such as the relatively narrow operating temperature window of 300–400 °C, low N2 selectivity and high conversion of SO2 to SO3 at high temperatures.4,6,7 Besides, the high concentration of ash containing K2O, CaO, As2O3 in the flue gas reduces the performance and longevity of V2O5–WO3(MoO3)/TiO2 catalysts.8,9 These could be avoided by locating the SCR unit downstream of the electrostatic precipitator unit and even downstream of the desulfurizer, through the development of a highly efficient low temperature SCR system.In our previous study, we have developed a VOx/CeO2 catalyst, prepared by a homogeneous precipitation method, showing excellent NH3-SCR activity, N2 selectivity and SO2 durability.10 However, the catalytic activity was not high enough for the application in the deNOx process of exhaust gas with low temperature, such as the flue gas after dust removal and desulfurization from coal-fired power plants. Therefore, it is very necessary to modify this vanadium–cerium catalyst to improve the low temperature activity, which is crucial for its practical utilization.
Niobium compound materials are of current interest as important catalysts for various reactions, such as the removal of nitrogen oxides, the hydrogenation and oxidative dehydrogenation of alkanes, acting as a catalyst promoter, catalyst support or solid acid catalyst.11 It was reported that when the niobium oxides were introduced into V2O5/TiO2 catalysts, the conversion of NO in NH3-SCR reaction increased 2–4 times at low temperatures.12 The addition of Nb to MnOx–CeO2 was also found to be very effective in improving the NH3-SCR activity and N2 selectivity.13,14 Mn2Nb1Ox catalyst exhibited higher NH3-SCR activity than MnOx catalyst.15 In addition, it was reported that the introduction of the third main group element (such as Mn, Fe, Co, Mo) could also improve the activity, stability or SO2 durability of the SCR catalysts.16–19 Therefore, based on our V–Ce catalyst, we can add other elements to adjust its physicochemical properties, expecting to enhance the low temperature SCR activity.
In this study, a series of M-VOx/CeO2 catalysts (M = Mn, Fe, Co, Nb, Mo) were prepared by the homogeneous precipitation method and were applied to the low-temperature NH3-SCR reaction. The addition of Nb could significantly promote the SCR activity over the VOx/CeO2 catalyst. Among the catalysts with different NbOx contents, VOx/CeO2 with 30 wt% NbOx catalyst showed the best catalytic performance and better SO2/H2O tolerance compared to VOx/CeO2 catalyst. The lower crystallinity, the stronger redox capability and the more Brønsted acid sites of the Nb-VOx/CeO2 catalyst were all responsible for its higher SCR activity.
2 Experiments
2.1 Catalyst synthesis and activity tests
The VOx/CeO2 oxide catalysts were prepared by a homogeneous precipitation method. Aqueous solutions of NH4VO3 (H2C2O4 was added to facilitate the dissolution of NH4VO3) and Ce(NO3)2 were mixed with the desired molar ratios (the mass ratio of vanadium oxide was controlled at 1 wt%). An excess of aqueous urea solution was then added to the mixed solution. The solution was heated to 90 °C and held there for 12 h under vigorous stirring. After filtration and washing with deionized water, the resulting precipitate was dried at 100 °C overnight and subsequently calcined at 350 °C for 3 h in air. M-VOx/CeO2 catalysts (the mass ratio of MOx were controlled at 30 wt%) were also prepared by homogeneous precipitation methods using Mn(NO3)2, Co(NO3)3, Fe(NO3)3, NbCl5, and (NH4)6Mo7O24 as precursors, respectively. Nb-VOx/CeO2 catalysts with different Nb contents (10, 30, 50 wt%) and the unpromoted 3 wt% VOx/CeO2 catalyst were also prepared by the same method. For comparison, the conventional 3 wt% V2O5-10 wt% WO3/TiO2 and 1 wt% V2O5-10 wt% WO3/TiO2 were prepared by impregnation method using NH4VO3, (NH4)10W12O41 as precursors and anatase TiO2 as support. After impregnation, the excess water was removed in a rotary evaporator at 60 °C. The samples dried at 100 °C overnight and then calcined at 500 °C for 3 h in air condition.Before NH3-SCR activity tests, the catalysts were pressed, crushed and sieved to 40–60 mesh. The SCR activity tests were carried out in a fixed-bed quartz flow reactor at atmospheric pressure. The reaction conditions were controlled as follows: 500 ppm NO, 500 ppm NH3, 5 vol% O2, 100 ppm SO2 (when used), 5 vol% H2O (when used), N2 balance. Under ambient conditions, the total flow rate was 500 ml min−1 and the gas hourly space velocity (GHSV) was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The amount of catalysts used in activity tests was 0.6 ml (about 0.7 g). The effluent gas including NO, NH3, NO2 and N2O was continuously analyzed by an FTIR spectrometer (Nicole Nexus 670) equipped with a heated, low-volume multiple-path gas cell (2m). The FTIR spectra were collected after the SCR reaction reached a steady state, and the NOx conversion and N2 selectivity were calculated as follows:
000 h−1. The amount of catalysts used in activity tests was 0.6 ml (about 0.7 g). The effluent gas including NO, NH3, NO2 and N2O was continuously analyzed by an FTIR spectrometer (Nicole Nexus 670) equipped with a heated, low-volume multiple-path gas cell (2m). The FTIR spectra were collected after the SCR reaction reached a steady state, and the NOx conversion and N2 selectivity were calculated as follows:
2.2 Characterization of catalysts
The surface area and pore characterization of the catalysts were obtained from N2 adsorption/desorption analysis at −196 °C using a Quantachrome Quadrasorb SI-MP. Prior to the N2 physisorption, the catalysts were degassed at 300 °C for 5 h. Surface areas were determined by the BET equation in the 0.05–0.35 partial pressure range. Pore volumes and average pore diameters were determined by the Barrett–Joyner–Halenda (BJH) method from the desorption branches of the isotherms.Powder X-ray diffraction (XRD) measurements of the catalysts were carried out on a computerized PANalytical X'Pert Pro diffractometer with Cu Kα (λ = 0.15406 nm) radiation. The data of 2θ from 10 to 80° were collected at 8° min−1 with the step size of 0.07°.
The H2-TPR experiments were carried out on a Micromeritics Auto Chem 2920 chemisorption analyzer. The samples (30 mg) were pretreated at 300 °C in a flow of 20 vol% O2/Ar (50 ml min−1) for 0.5 h in a quartz reactor and cooled down to room temperature (30 °C) followed by Ar purging for 0.5 h. A 50 ml min−1 gas flow of 10% H2 in Ar was then passed over the samples through a cold trap to the detector. The reduction temperature was raised at 10 °C min−1 from 30 to 1000 °C.
2.3 NH3-TPD studies
NH3-TPD experiments were performed in the same instrument as the H2-TPR, equipped with a quadrupole mass spectrometer (MKS Cirrus) to record the signals of NH3 (m/z = 17 for NH3, the interference of H2O was eliminated by using a cold trap before the detector). Prior to TPD experiments, the samples (100 mg) were pretreated at 300 °C in a flow of 20 vol% O2/Ar (50 ml min−1) for 0.5 h and cooled down to room temperature (30 °C). The samples were then exposed to a flow of 2500 ppm NH3/Ar (50 ml min−1) at 30 °C for 1 h, followed by Ar purging for another 1 h. Finally, the temperature was raised to 600 °C in Ar flow at the rate of 10 °C min−1.2.4 In situ DRIFTS studies
In situ DRIFTS experiments were performed on an FTIR spectrometer (Nicolet Nexus 670) equipped with a smart collector and an MCT/A detector cooled by liquid nitrogen. The reaction temperature was controlled precisely by an Omega programmable temperature controller. Prior to each experiment, the sample was pretreated at 300 °C for 0.5 h in a flow of 20 vol% O2/N2 and then cooled down to 175 °C. The background spectra were collected in flowing N2 and automatically subtracted from the sample spectrum. The reaction conditions were controlled as follows: 200 ml min−1 total flow rate, 500 ppm NH3 or/and 500 ppm NO + 5 vol% O2, and N2 balance. All spectra were recorded by accumulating 100 scans with a resolution of 4 cm−1.3 Results and discussion
3.1 Catalytic performance
 | ||
Fig. 2 NH3-SCR activity over Nb-VOx/CeO2 catalysts. Reaction conditions: [NO] = [NH3] = 500 ppm, [O2] = 5 vol%, N2 balance, total flow rate 500 ml min−1 and GHSV = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. 000 h−1. | ||
To better evaluate the NH3-SCR activity over Nb-VOx/CeO2 catalyst, we also carried out the comparative SCR activity test over V2O5–WO3/TiO2 (Fig. 3). 30 wt% Nb-1VOx/CeO2 exhibited higher NOx conversion than 1VOx/CeO2 and 3VOx/CeO2. However, there was not notable enhancement over 30Nb-3VOx/CeO2 in contrast to 3VOx/CeO2 (as shown in Fig. S3†). Compared to 3VOx/CeO2, the 30Nb-1VOx/CeO2 catalyst not only decreased the content of vanadium oxide, but also enhanced the catalytic activity. The NH3-SCR performance over 30Nb-1VOx/CeO2 catalyst was also better than that over 3V2O5–10WO3/TiO2 and 1V2O5–10WO3/TiO2. The NOx conversion over three catalysts at 175 °C was 96%, 63% and 15%, respectively. Therefore, 30Nb-1VOx/CeO2 showed excellent NH3-SCR performance.
When 5% H2O was introduced to the inlet gas, the NOx conversion over VOx/CeO2 decreased rapidly to 56% and kept in 56% in 48 h test. The catalytic activity could recover to the original level after the removal of H2O, indicating that the poison of H2O was reversible. Meanwhile, H2O had no influence on the catalytic activity over Nb-VOx/CeO2 catalyst and 100% NOx conversion was maintained all the time. Nb-VOx/CeO2 exhibited much higher catalytic activity and stronger resistance to SO2/H2O than VOx/CeO2.
3.2 Catalyst characterization
| Catalysts | Specific surface area (m2 g−1) | Pore diameter (nm) | Pore volume (cm−3 g−1) |
|---|---|---|---|
| 1VOx/CeO2 | 131.3 | 3.50 | 0.11 |
| 30Nb-1VOx/CeO2 | 168.2 | 3.48 | 0.15 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The intensity of the low temperature (380 °C) reduction peak of Nb-VOx/CeO2 was much stronger than that of VOx/CeO2 (1.44
1). The intensity of the low temperature (380 °C) reduction peak of Nb-VOx/CeO2 was much stronger than that of VOx/CeO2 (1.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This could be due to the interaction of V, Ce and Nb over Nb-VOx/CeO2 catalyst resulting in better dispersion of vanadium species and stronger redox capability. The active temperature window in NH3-SCR reaction was between 150–400 °C. Stronger redox capability in this temperature range could enhance NH3-SCR performance. Therefore, Nb doped VOx/CeO2 showed higher NH3-SCR activity.
1). This could be due to the interaction of V, Ce and Nb over Nb-VOx/CeO2 catalyst resulting in better dispersion of vanadium species and stronger redox capability. The active temperature window in NH3-SCR reaction was between 150–400 °C. Stronger redox capability in this temperature range could enhance NH3-SCR performance. Therefore, Nb doped VOx/CeO2 showed higher NH3-SCR activity.
3.3 NH3-TPD
Fig. 7 shows NH3-TPD results over 1VOx/CeO2 and 30Nb-1VOx/CeO2 catalysts using the fragment of m/z = 17 to identify NH3. There were two NH3 desorption peaks around 100 and 250 °C on both catalysts. It is generally accepted that NH4+ ions bound to Brønsted acid sites are less thermally stable than coordinated NH3 molecules bound to Lewis acid sites and desorb at lower temperatures.18,23,24 Therefore, the desorption peak at 100 °C could be ascribed to the desorption of physisorbed NH3 and the partial ionic NH4+ bound to the weak Brønsted acid sites, and the peak at 250 °C could be assigned to the desorption of ionic NH4+ bound to strong Brønsted acid sites and coordinated NH3 bound to the Lewis acid sites. The amount of acid sites over Nb-VOx/CeO2 was remarkably larger than that of VOx/CeO2 (1.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It indicates that Nb-VOx/CeO2 catalyst presented more acid sites which could facilitate the adsorption and activation of NH3 during catalytic reaction even in the presence of H2O and SO2 thus enhances the catalytic activity in NH3-SCR. In the presence of SO2, more acid sites over Nb-VOx/CeO2 catalyst inhibited the adsorption of SO2 and the deposition of sulfate thus enhanced the resistance to SO2. More acid sites could also promote the adsorption of NH3 in the presence of H2O and reduce the influence of competitive adsorption of H2O with NH3. Furthermore, larger specific surface area was obtained over Nb-VOx/CeO2. Therefore, Nb-VOx/CeO2 catalyst showed stronger resistance to H2O/SO2.
1). It indicates that Nb-VOx/CeO2 catalyst presented more acid sites which could facilitate the adsorption and activation of NH3 during catalytic reaction even in the presence of H2O and SO2 thus enhances the catalytic activity in NH3-SCR. In the presence of SO2, more acid sites over Nb-VOx/CeO2 catalyst inhibited the adsorption of SO2 and the deposition of sulfate thus enhanced the resistance to SO2. More acid sites could also promote the adsorption of NH3 in the presence of H2O and reduce the influence of competitive adsorption of H2O with NH3. Furthermore, larger specific surface area was obtained over Nb-VOx/CeO2. Therefore, Nb-VOx/CeO2 catalyst showed stronger resistance to H2O/SO2.
3.4 In situ DRIFTS studies
To investigate NH3/NOx adsorption on 1VOx/CeO2 and 30Nb-1VOx/CeO2 catalysts together with the SCR reaction mechanism, in situ DRIFTS were conducted at 175 °C and the results of NH3 adsorption on VOx/CeO2 and Nb-VOx/CeO2 catalysts are shown in Fig. 8A. After NH3 adsorption and N2 purge, both catalysts were covered by different NH3 species. The bands at 1423 and 1670 cm−1 were assigned to asymmetric and symmetric bending vibrations of ionic NH4+ on Brønsted acid sites and the bands at 1596 (1604) and 1147 (1200) cm−1 were attributed to asymmetric and symmetric bending vibrations of the N–H bonds in NH3 coordinately linked to the Lewis acid sites.25–27 Nb-VOx/CeO2 catalyst provided more acid sites than VOx/CeO2 (1.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), especially the Brønsted acid sites, than VOx/CeO2, which was in well harmony with the NH3-TPD results. As shown in our previous paper (Chem. Eng. J., 2014, 250, 390–398),15 the NbOx itself did not show any NH3-SCR activity in the whole temperature range that we investigated. However, the addition of Nb to 1VOx/CeO2 increased the surface acidity. Nb mainly played as acid sites for the promotion of NH3 adsorption in NH3-SCR reaction.
1), especially the Brønsted acid sites, than VOx/CeO2, which was in well harmony with the NH3-TPD results. As shown in our previous paper (Chem. Eng. J., 2014, 250, 390–398),15 the NbOx itself did not show any NH3-SCR activity in the whole temperature range that we investigated. However, the addition of Nb to 1VOx/CeO2 increased the surface acidity. Nb mainly played as acid sites for the promotion of NH3 adsorption in NH3-SCR reaction.
 | ||
| Fig. 8 DRIFT spectra of 500 ppm NH3 adsorption (A) and 500 ppm NO + 5 vol% O2 adsorption (B) on VOx/CeO2 and Nb-VOx/CeO2 catalysts. | ||
The in situ DRIFT spectra of NO + O2 adsorption on 1VOx/CeO2 and 30Nb-1VOx/CeO2 catalysts at 175 °C were also conducted, and the results are shown in Fig. 8B. When VOx/CeO2 catalyst was exposed to NO + O2, bands assigned to nitrate species were observed including monodentate nitrate (1540 and 1270 cm−1), bridging nitrate (1215 and 1592 cm−1) and bidentate nitrate (1570 and 1243 cm−1).28–30 On the Nb-VOx/CeO2 catalyst, the adsorption amount of NOx was larger than that on the VOx/CeO2 catalyst (1.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The addition of Nb to the VOx/CeO2 catalyst increased the acidity but did not inhibit the adsorption of NOx on the catalyst surface simultaneously. It could be due to its stronger oxidation at low temperature and its larger specific surface area. Therefore, the Nb-VOx/CeO2 catalyst produced more nitrate species than VOx/CeO2.
1). The addition of Nb to the VOx/CeO2 catalyst increased the acidity but did not inhibit the adsorption of NOx on the catalyst surface simultaneously. It could be due to its stronger oxidation at low temperature and its larger specific surface area. Therefore, the Nb-VOx/CeO2 catalyst produced more nitrate species than VOx/CeO2.
According to the Arrhenius equation, the activation energy over VOx/CeO2 and Nb-VOx/CeO2 catalysts was calculated as 47 and 38 kJ mol−1 (as shown in Fig. S5†). The interaction of V, Ce and Nb of the Nb-VOx/CeO2 catalyst decreased the activation energy of NO reduction and promote the NH3-SCR reaction.
To investigate the reactivity of adsorbed NH3 species in the SCR reaction on 30Nb-1VOx/CeO2 catalysts, the in situ DRIFTS of reaction between pre-adsorbed NH3 and NO + O2 at 175 °C were recorded as a function of time (Fig. 9A). After NH3 pre-adsorption and N2 purge, the catalyst surface was covered by the adsorbed NH3 species. When NO + O2 was introduced, the intensity of the bands attributed to NH3 species decreased and disappeared after 10 min. At the same time, the bands assigned to nitrate species (monodentate nitrate at 1540 cm−1, bridging nitrate at 1215, 1592 cm−1 and bidentate nitrate at 1570, 1243 cm−1) appeared. This result suggested that both ionic NH4+ and coordinated NH3 could react with NOx and participate in the NH3-SCR reactions. Fig. S6† showed the band intensities of adsorbed NH3 species over VOx/CeO2 and 30Nb-VOx/CeO2 pretreated by exposure to NH3 followed by exposure to NO + O2 at 175 °C. The reactive rate of adsorbed NH3 species with gas NO and O2 over Nb-VOx/CeO2 was much higher than that over VOx/CeO2. Therefore, more adsorbed NH3 species on Nb-VOx/CeO2 contributed to its better SCR activity.
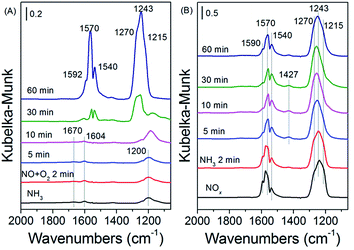 | ||
| Fig. 9 In situ DRIFT spectra over Nb-VOx/CeO2 pretreated by exposure to NO + O2 followed by exposure to NH3 at 175 °C (A), and by exposure to NH3 followed by exposure to NO + O2 at 175 °C (B). | ||
The reaction between the pre-adsorbed NOx and NH3 on 30Nb-1VOx/CeO2 catalysts was also investigated by the in situ DRIFTS, and the results were shown in Fig. 9B. After NO + O2 pre-adsorption and N2 purge, Nb-VOx/CeO2 catalyst surface was covered by various nitrate species. When NH3 was introduced, the intensity of bridging nitrate decreased, which indicated that adsorbed NOx species could also react with NH3. NH3 adsorbed species were observed at the band of 1427 cm−1.
The kinetic experiments were also carried out to investigate the reaction order and the tests met the condition of differential reactor model. The NO conversion data in the kinetics test were in differential regime (conversion less than 20%) (as shown in Fig. S7†). A relative small particles size (40–60 mesh) and the volume hourly space velocity (about 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1)31 ensured the elimination of internal and external diffusion, respectively. The rates of NO conversion increased linearly with NO concentration over 30Nb-1VOx/CeO2 catalyst (as shown in Fig. S8†), and the reaction order for NO was calculated as 0.555. The reaction order for NO was lower than 1, indicating the presence of Langmuir–Hinshelwood mechanism, which was in agreement with DRIFTS results. In Fig. 9B, the intensity of the bands attributed to nitrate species weakened after exposure to NH3 indicating that adsorbed NOx species could react with adsorbed NH3 to finally form N2 and H2O. In our previous study,10 for the 3VOx/CeO2 catalysts pre-adsorbed NOx species, when NH3 was introduced, the intensity of the bands attributed to monodentate nitrate and bridging nitrate species decreased slightly. The amount of bidentate nitrate species increased markedly, which may be due to the transformation of monodentate and bridging nitrate to bidentate nitrate (Fig. 10 (ref. 10) and S5†10). The adsorbed nitrate species were mostly inactive in the NH3-SCR reaction and therefore the 3VOx/CeO2 catalysts mainly followed the Eley–Rideal mechanism.
000 h−1)31 ensured the elimination of internal and external diffusion, respectively. The rates of NO conversion increased linearly with NO concentration over 30Nb-1VOx/CeO2 catalyst (as shown in Fig. S8†), and the reaction order for NO was calculated as 0.555. The reaction order for NO was lower than 1, indicating the presence of Langmuir–Hinshelwood mechanism, which was in agreement with DRIFTS results. In Fig. 9B, the intensity of the bands attributed to nitrate species weakened after exposure to NH3 indicating that adsorbed NOx species could react with adsorbed NH3 to finally form N2 and H2O. In our previous study,10 for the 3VOx/CeO2 catalysts pre-adsorbed NOx species, when NH3 was introduced, the intensity of the bands attributed to monodentate nitrate and bridging nitrate species decreased slightly. The amount of bidentate nitrate species increased markedly, which may be due to the transformation of monodentate and bridging nitrate to bidentate nitrate (Fig. 10 (ref. 10) and S5†10). The adsorbed nitrate species were mostly inactive in the NH3-SCR reaction and therefore the 3VOx/CeO2 catalysts mainly followed the Eley–Rideal mechanism.
4 Conclusions
A systematic study on the effect of Nb addition to the VOx/CeO2 catalyst for the low-temperature NH3-SCR reaction was carried out. VOx/CeO2 and Nb doped VOx/CeO2 catalysts were prepared by homogeneous precipitation method and the SCR activity at low temperature was enhanced by the addition of Nb. The NH3-SCR activity over 1%VOx/CeO2 with 30 wt% NbOx catalyst were higher than that over the 3%V2O5–WO3/TiO2 catalyst. 30Nb-1VOx/CeO2 showed higher catalytic activity than 1VOx/CeO2 catalyst, due to the weaker crystallinity, the stronger redox capability and the more Brønsted acid sites. The excellent SO2/H2O tolerance was also obtained over 30Nb-1VOx/CeO2 catalyst. The Langmuir–Hinshelwood mechanism existed for selective catalytic reduction of NO over 30Nb-1VOx/CeO2, in which adsorbed NOx species reacted with adsorbed NH3 to finally form N2 and H2O.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51221892) and the Ministry of Science and Technology, China (2013AA065301).References
- G. S. Qi, R. T. Yang and R. Chang, Appl. Catal., B, 2004, 51, 93 CrossRef CAS PubMed.
- H. Bosch and F. Janssen, Catal. Today, 1988, 2, 369 CrossRef CAS.
- Z. G. Huang, Z. P. Zhu, Z. Y. Liu and Q. Y. Liu, J. Catal., 2003, 214, 213 CrossRef CAS.
- G. Busca, L. Lietti, G. Ramis and F. Berti, Appl. Catal., B, 1998, 18, 1 CrossRef CAS.
- G. Busca, M. A. Larrubia, L. Arrighi and G. Ramis, Catal. Today, 2005, 107–108, 139 CrossRef CAS PubMed.
- J. P. Dunn, P. R. Koppula, H. G. Stenger and I. E. Wachs, Appl. Catal., B, 1998, 19, 103 CrossRef CAS.
- P. Balle, B. Geiger and S. Kureti, Appl. Catal., B, 2009, 85, 109 CrossRef CAS PubMed.
- I. E. Wachs and B. M. Weckhuysen, Appl. Catal., A, 1997, 157, 67 CrossRef CAS.
- D. A. Bulushev, F. Rainone, L. Kiwi-Minsker and A. Renken, Langmuir, 2001, 17, 5276 CrossRef CAS.
- Z. Lian, F. Liu and H. He, Catal. Sci. Technol., 2015, 5, 389 CAS.
- K. Tanabe, Catal. Today, 2003, 78, 65 CrossRef CAS.
- K. A. Vikulov, A. Andreini, E. K. Poels and A. Bliek, Catal. Lett., 1994, 25, 49 CrossRef CAS.
- M. Casapu, O. Krocher, M. Mehring, M. Nachtegaal, C. Borca, M. Harfouche and D. Grolimund, J. Phys. Chem. C, 2010, 114, 9791 CAS.
- M. Casapu, O. Kröcher and M. Elsener, Appl. Catal., B, 2009, 88, 413 CrossRef CAS PubMed.
- Z. Lian, F. Liu, H. He, X. Shi, J. Mo and Z. Wu, Chem. Eng. J., 2014, 250, 390 CrossRef CAS PubMed.
- F. Liu, H. He, Y. Ding and C. Zhang, Appl. Catal., B, 2009, 93, 194 CrossRef CAS PubMed.
- X. Li and Y. Li, J. Mol. Catal. A: Chem., 2014, 386, 69 CrossRef CAS PubMed.
- S. Roy, B. Viswanath, M. S. Hedge and G. Madras, J. Phys. Chem. C, 2008, 112, 6002 CAS.
- Z. Liu, S. Zhang, J. Li and L. Ma, Appl. Catal., B, 2014, 144, 90 CrossRef CAS PubMed.
- Y. Peng, J. H. Li, L. Chen, J. H. Chen, J. Han, H. Zhang and W. Han, Environ. Sci. Technol., 2012, 46, 2864 CrossRef CAS PubMed.
- S. J. Yang, Y. F. Guo, H. Z. Chang, L. Ma, Y. Peng, Z. Qu, N. Q. Yan, C. Z. Wang and J. H. Li, Appl. Catal., B, 2013, 136, 19 CrossRef PubMed.
- S. Youn, S. Jeong and D. H. Kim, Catal. Today, 2014, 232, 185 CrossRef CAS PubMed.
- R. B. Jin, Y. Liu, Z. B. Wu, H. Q. Wang and T. T. Gu, Chemosphere, 2010, 78, 1160 CrossRef CAS PubMed.
- K. J. Lee, M. S. Maqbool, P. A. Kumar, K. H. Song and H. P. Ha, Catal. Lett., 2013, 143, 988 CrossRef CAS PubMed.
- R. Gao, D. Zhang, X. Liu, L. Shi, P. Maitarad, H. Li, J. Zhang and W. Cao, Catal. Sci. Technol., 2013, 3, 191 CAS.
- L. Zhang, J. Pierce, V. L. Leung, D. Wang and W. S. Epling, J. Phys. Chem. C, 2013, 117, 8282 CAS.
- K. J. Lee, P. A. Kumar, M. S. Maqbool, K. N. Rao, K. H. Song and H. P. Ha, Appl. Catal., B, 2013, 142, 705 CrossRef PubMed.
- K. I. Hadjiivanov, Catal. Rev.: Sci. Eng., 2000, 42, 71 CAS.
- C. Liu, L. Chen, J. Li, L. Ma, H. Arandiyan, Y. Du, J. Xu and J. Hao, Environ. Sci. Technol., 2012, 46, 6182 CrossRef CAS PubMed.
- Z. Si, D. Weng, X. Wu, Z. Ma, J. Ma and R. Ran, Catal. Today, 2013, 201, 122 CrossRef CAS PubMed.
- R. Raj, M. P. Harold and V. Balakotaiah, Ind. Eng. Chem. Res., 2013, 52, 15455 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02752g |
| ‡ Present address: Materials Sciences Division, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720, USA. |
| This journal is © The Royal Society of Chemistry 2015 |








