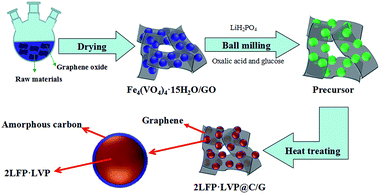
High-rate electrode material 2LiFePO4·Li3V2(PO4)3@carbon/graphene using the in situ grown Fe4(VO4)4·15H2O precursor on the surface of graphite oxide
Bao Zhang,
Hui Li and
Jia-feng Zhang*
School of Metallurgy and Environment, Central South University, Changsha, Hunan 410083, China. E-mail: csuzjf@vip.163.com; Tel: +86-731-88877248
First published on 31st March 2015
Abstract
2LiFePO4·Li3V2(PO4)3@carbon/graphene (2LFP·LVP@C/G) as a cathode material, based on an in situ grown Fe4(VO4)4·15H2O precursor on the surface of graphene oxide, was synthesized by a solid-state process. The X-ray diffraction Rietveld refinement and Raman spectroscopy results indicated that multi-phase structural 2LiFePO4·Li3V2(PO4)3 with a carbon/graphene coating was obtained. The morphology is characterized by HRTEM tests, which reveal well crystallized 2LFP·LVP@C/G with bridging graphene nanosheets, forming an effective three-dimensional conducting network. Compared with 2LiFePO4·Li3V2(PO4)3@carbon (2LFP·LVP@C), the electrochemical results demonstrate that the 2LFP·LVP@C/G electrode measured at 0.1 C and 10 C can deliver a high specific discharge capacity of 151.2 mA h g−1 and 125.4 mA h g−1, respectively, and has a discharge capacity of 100.2 mA h g−1 at −30 °C at 0.1 C, indicating better rate capability and thermal properties.
1 Introduction
Since Padhi et al.1 first reported that lithium iron phosphate was a potential cathode for lithium ion batteries, many studies have been devoted to preparing transition metal polyanion materials based on PO43−, such as orthorhombic LiMPO4 (M = Fe, Mn, Co, Ni)2,3 and monoclinic Li3M′2(PO4)3 (M′ = Fe, V).4,5 Olivine-type LiFePO4 is one of the most promising cathode materials owing to the advantages of abundant resources, stable structures, high power density and nontrivial safety. However, LiFePO4 has inherently poor electrical conductivity (1.8 × 10−9 S cm−1) and ionic conductivity (10−16 to 10−14 S cm−1), which hinder its practical applications.6,7 The presence of the tetrahedral poly-anion (PO43−) with strong covalently-bond in the olivine structure could provide a steady three-dimensional framework, thus retaining the excellent cycling performance and thermal stability for Li3V2(PO4)3 cathode materials.8 Compared with LiFePO4, monoclinic Li3V2(PO4)3 possesses superior electrical conductivity (2.4 × 10−7 S cm−1) and ionic conductivity (10−9 to 10−8 S cm−1), belonging to the lithium high ionic conductors with NASCION structure, which enables Li ions to intercalate and deintercalate effortlessly.8–10 Therefore, the ionic diffusivity of LiFePO4 could be improved by incorporating with Li3V2(PO4)3 and the xLiFePO4·yLi3V2(PO4)3 compound is a novel strategy for enhancing the electrochemical performance of LiFePO4.11–31 Many mixtures of LiFePO4 and Li3V2(PO4)3 have been studied and delivered excellent rate performances,32,33 such as, 100 mA h g−1 at 10 C for 2LiFePO4/C–Li3V2(PO4)3/C,34 116.8 mA h g−1 at 10 C for 3LiFePO4/C–Li3V2(PO4)3/C,35 114 mA h g−1 at 10 C for 5LiFePO4–Li3V2(PO4)3,36 93.3 mA h g−1 at 10 C for 9LiFePO4/C–Li3V2(PO4)3/C37 etc.Recently, graphene, with one-atom thick layer in 2D structure,38 has been implemented as a new and promising electron conducting additive for cathode materials of LIBs. Graphene can offer an improved interfacial contact because of its superior conductivity, flexible structure, especially, high surface area (2630 m2 g−1 of theoretical value),39 which can form a 2D electron conducting network in cathode materials to increase electron conductivity.40 Ding et al. reported LiFePO4/graphene composites prepared through a co-precipitation method,41 and Zhou et al. synthesized graphene modified LiFePO4 composites with excellent electrochemical properties by spray-drying and annealing processes.42 Liu et al. prepared Li3V2(PO4)3/graphene composite with a high specific discharge capacity of 82 mA h g−1 at 50 C rate in the potential range of 3.0–4.3 V.43
However, the interfacial interaction between cathode materials and graphene is inefficiency and graphene was difficult to be uniformly dispersed, which is led to quite limitation about the enhancement in electrical conductivity. Consequently, it remains necessary to develop an approach to construct effective graphene networks for fully utilizing its potential properties.
In this paper, a novel in situ growing Fe4(VO4)4·15H2O precursor on the surface of graphite oxide are developed to a novel 3D hierarchical 2LiFePO4·Li3V2(PO4)3@carbon/graphene (2LFP·LVP@C/G) composite cathode in situ for the first time. The composite showed enhanced electrochemical performance as cathode material for Li-ion battery compared with 2LiFePO4·Li3V2(PO4)3@C (2LFP·LVP@C).
2 Experimental
The precursor Fe4(VO4)4·15H2O/GO was prepared by ultrasonic-aided precipitation method. Firstly, graphene oxide (GO) was prepared from graphite powder (Aldrich, powder, b20 μm, synthetic) according to the method reported by Hummers.44 Then, a 2 M aqueous solution of Fe(NO3)3·9H2O and GO (the weight ratio of GO to Fe4(VO4)4·15H2O was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) were mixed in an ultrasonic-aided reaction vessel under vigorous stirring at 60 °C for 24 h. Then, the desired amount of NH4VO3 was added drop wise under a continuous stirring. The molar ratio of Fe
90) were mixed in an ultrasonic-aided reaction vessel under vigorous stirring at 60 °C for 24 h. Then, the desired amount of NH4VO3 was added drop wise under a continuous stirring. The molar ratio of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V was 1
V was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Meanwhile, NH3·H2O was employed to adjust the pH of the reaction solution to 6. As we expected, a brown precipitate Fe4(VO4)4·xH2O/GO was spontaneously appeared, finally the precipitate was collected after washing by deionized water and dried in an oven at 80 °C. The possible reaction may occur as follow:
1. Meanwhile, NH3·H2O was employed to adjust the pH of the reaction solution to 6. As we expected, a brown precipitate Fe4(VO4)4·xH2O/GO was spontaneously appeared, finally the precipitate was collected after washing by deionized water and dried in an oven at 80 °C. The possible reaction may occur as follow:| V4O124− + 4Fe3+ + 8OH− + 15H2O + GO → Fe4(VO4)4·15H2O/GO |
The 2LFP·LVP@C/G was prepared by mixing stoichiometric amounts of as-prepared Fe4(VO4)4·15H2O/GO, LiH2PO4, oxalic acid and glucose in ethanol medium under high-energy ball milling for 5 h. The as-obtained powders were sintered in a horizontal quartz tube at 700 °C for 18 h under a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 hydrogen–argon atmosphere, then the 2LFP·LVP@C/G composites were finally obtained. The synthesis process for the 2LFP·LVP/G composites is schematically illustrated in Fig. 1. Meanwhile, the material 2LFP·LVP@C was prepared via the same process without using graphene oxide as precursor for comparison.
95 hydrogen–argon atmosphere, then the 2LFP·LVP@C/G composites were finally obtained. The synthesis process for the 2LFP·LVP/G composites is schematically illustrated in Fig. 1. Meanwhile, the material 2LFP·LVP@C was prepared via the same process without using graphene oxide as precursor for comparison.
Structural and crystalline phase analyses of the products were taken from the powder X-ray diffraction (XRD, Rint-2000, Rigaku) using Cu Kα. Residual carbon content in both samples was determined by a carbon sulfur analyzer (CS600, LECO, US). The samples were observed by SEM (JEOL, JSM-5600LV) and a Tecnai G12 transmission electron microscope (TEM). Thermogravimetric (TG) analysis of the precursor was measured on a SDT Q600 TG-DTA apparatus at the temperature between 25 °C and 800 °C with a heating rate of 10 °C min−1 under air flow. Raman scattering (RS) spectra were recorded on a HORIBA Scientific LabRAM HR Raman spectrometer system.
The electrochemical characterizations were performed using CR2025 coin-type cell. Typical positive electrode loadings were in the range of 2–2.5 mg cm−2, and an electrode diameter of 14 mm was used. For positive electrode fabrication, the prepared powders were mixed with 10% of carbon black and 10% of polyvinylidene fluoride in N-methyl pyrrolidinone until slurry was obtained. And then, the blended slurries were pasted onto an aluminum current collector, and the electrode was dried at 120 °C for 12 h in the air. The test cell consisted of the positive electrode and lithium foil negative electrode separated by a porous polypropylene film, and 1.0 mol L−1 (0.9LiBF4–0.1LiBOB) in PC–EC–EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) was used as the electrolyte. The assembly of the cells was carried out in a dry Ar-filled glove box. Electrochemical tests were carried out using an automatic galvanostatic charge–discharge unit, NEWARE battery cycler, between 2.5 V and 4.5 V versus Li/Li+ electrode at room temperature. Cyclic voltammograms (CV) were measured on a LK9805 electrochemical interface at a scanning rate of 0.1 mV s−1. Electrochemical impedance spectroscopy (EIS) was conducted using an electrochemical analyzer (Zahner) at frequencies from 100 kHz to 10 mHz with a perturbation amplitude.
1 in volume) was used as the electrolyte. The assembly of the cells was carried out in a dry Ar-filled glove box. Electrochemical tests were carried out using an automatic galvanostatic charge–discharge unit, NEWARE battery cycler, between 2.5 V and 4.5 V versus Li/Li+ electrode at room temperature. Cyclic voltammograms (CV) were measured on a LK9805 electrochemical interface at a scanning rate of 0.1 mV s−1. Electrochemical impedance spectroscopy (EIS) was conducted using an electrochemical analyzer (Zahner) at frequencies from 100 kHz to 10 mHz with a perturbation amplitude.
3 Results and discussion
The TG/DTA curves of Fe4(VO4)4·15H2O/GO compounds are shown in Fig. 2a. It can be seen that the main weight loss is up to 11.5% from 25 °C to 370 °C in the TG curve and two endothermic peaks occur at 113 °C and 369 °C in the DTA curve, indicating that the number of crystal water in the Fe4(VO4)4·15H2O/GO is 15. Then, an exothermic peak appeared in the DTA curve at 409 °C is ascribe to loss of GO (5.5%). Meanwhile, a litter exothermic peak appeared in the DTA curve at 462 °C corresponds to the decomposition of Fe4(VO4)4 and the crystal transformation of FeVO4. The XRD patterns of Fe4(VO4)4 and FeVO4 are shown in Fig. 2b. The morphology of Fe4(VO4)4·15H2O/GO was characterized by SEM. As we can see from Fig. 2c, the particles of Fe4(VO4)4·15H2O grow on the surfaces of graphene oxide. Additionally, the Fe/V mole ratio of Fe4(VO4)4·15H2O is 1.002 based on analysis of chemical titration, which confirms that the as prepared brown precipitate is Fe4(VO4)4·15H2O/GO.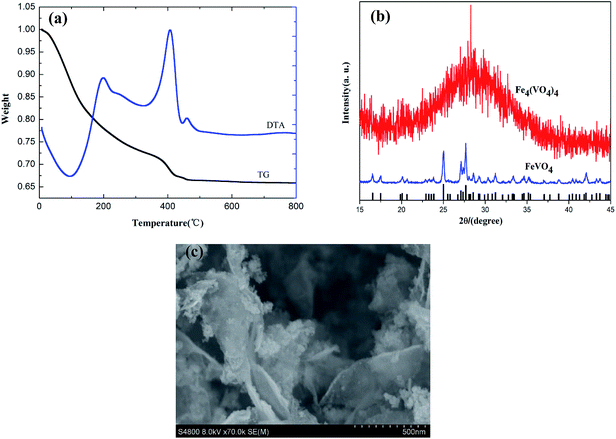 | ||
| Fig. 2 (a) TG-DTA curves of Fe4(VO4)4·15H2O/GO. (b) XRD patterns of Fe4(VO4)4 and FeVO4. (c) SEM images of Fe4(VO4)4·15H2O/GO. | ||
The XRD patterns of 2LFP·LVP@C/G and 2LFP·LVP@C are shown in Fig. 3, it indicates that two different 2LiFePO4·Li3V2(PO4)3 composites are all composed of orthorhombic LiFePO4 (space group Pnmb, PDF #83-20921) and monoclinic Li3V2(PO4)3 (space group P21/n, PDF #78-1465) phases without other impurities. There is no evidence of diffraction peaks for the carbon in all samples, indicating graphene. The Rietveld refinement result indicates the sample consists of two phases: LFP [46.5% w/w] and LVP [53.5% w/w]. The residual carbon contents of 2LFP·LVP@C/G and 2LFP·LVP@C measured using carbon sulfur analysis are 13.7 wt% and 14.0 wt%, respectively. The weight ratio of amorphous carbon to graphene in 2LFP·LVP@C/G composite is calculated to be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
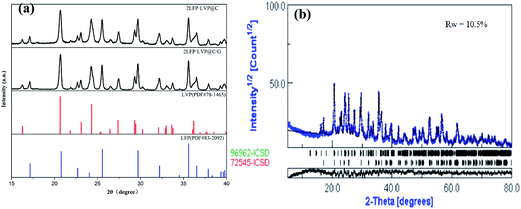 | ||
| Fig. 3 (a) XRD patterns of 2LFP·LVP@C/G and 2LFP·LVP@C. (b) Rietveld refinement performed with X-ray diffraction pattern of 2LFP·LVP@C/G. | ||
The TEM images of 2LFP·LVP@C/G is shown in Fig. 4. It can be seen from Fig. 4a that 2LFP·LVP@C/G nanoparticles with the size of about 180 nm are anchored on the surface of graphene sheets, which have tight contact surface with flexible graphene sheets. The Fig. 4b reflects the HRTEM images of 2LFP·LVP@C/G. It is found that there are two kinds of lattice fringes in the composite, one is the lattice fringe of LiFePO4 with an interplanar spacing of 0.278 nm, which corresponds to the (0 3 1) lattice planes, and the other is lattice fringe of Li3V2(PO4)3 with an interplanar spacing of 0.238 nm, which corresponds to the (1 4 2) lattice plane. The results imply that LiFePO4 unit cell, Li3V2(PO4)3 unit cell and 2LiFePO4·Li3V2(PO4)3 unit cell coexist in the composite materials.
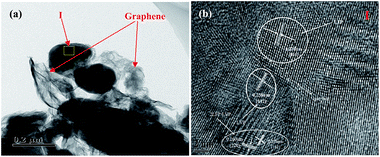 | ||
| Fig. 4 (a) TEM image of 2LFP·LVP@C/G particles, (b) corresponding HRTEM images from the marked region I. | ||
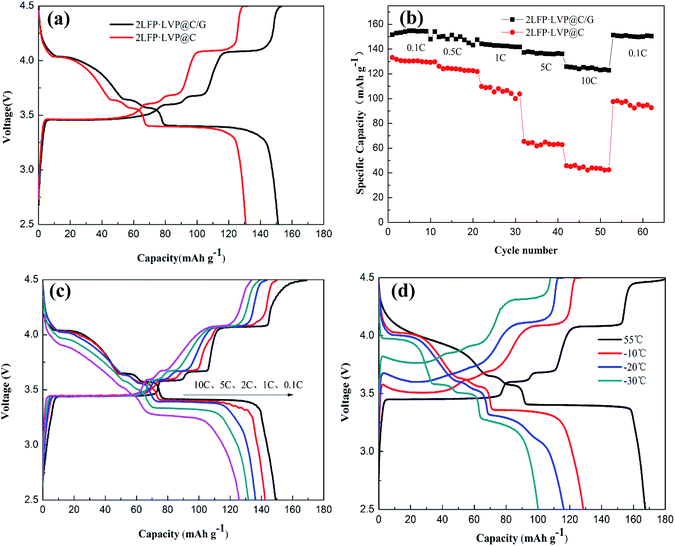
Fig. 5a shows the first charge–discharge curves of the different 2LFP·LVP@C samples at 0.1 C rate. At such rate, 2LFP·LVP@C/G exhibited the optimal initial discharge capacity of 152.1 mA h g−1 with a average voltage of 3.66 V over a voltage of 2.5–4.5 V, while 2LFP·LVP@C are only 130.7 mA h g−1 with a average voltage of 3.58 V over a voltage of 2.5–4.5 V, indicating unique structure (particles growth on grapheme) has positive effects on the electrochemical performances. To the best of our knowledge, the graphene oxide consists of sufficient oxygen containing groups, such as carboxyl, hydroxyl, and epoxide groups,45 which provide sites for the adsorption of Fe3+ and nanoparticles nucleation to achieve uniform Fe4(VO4)4 on the surface of graphene oxide. The resulting nanoparticles could be bonded to the partial GO by Fe–O–C bonds at the remaining oxygen sites and by van der Waals interactions with the aromatic regions of the partial GO.
Fig. 5b shows cycle performance of 2LFP·LVP@C/G and 2LFP·LVP@C at different rates. The cells were cycled at different rates from 0.1 C to 10 C and then returned to 0.1 C (10 cycles for each rate). It can be clearly seen that the discharge capacity of both samples gradually decreases with increasing rate because of the increased polarization and the decreased utilization of active materials at high current density. However, the highly stable reversible capacity of 125.4 mA h g−1 has been obtained at 10 C with a average voltage of 3.50 V over a voltage of 2.5–4.5 V for 2LFP·LVP@C/G compared with only a value of 44.5 mA h g−1 for LFP·LVP@C. Moreover, it is remarkable that the 2LFP·LVP@C/G electrode still remains a good discharge capacity of 151.2 mA h g−1 at 0.1 C after 50 cycles, which shows better electrochemical performance.
The rate capability of 2LFP·LVP@C/G at different rates is presented in Fig. 5c. The discharge capacities of the composites at the different rate of 0.1 C, 1 C, 2 C, 5 C and 10 C are 152.1 mA h g−1, 142.4 mA h g−1, 136.6 mA h g−1, 131.7 mA h g−1 and 126.2 mA h g−1, respectively. Fig. 5d shows high and low temperature property of 2LFP·LVP@C/G. The thermal and chemical stabilities of graphene guarantee the safety when it is used in harsh environments. The discharge capacities of the composites at the temperature of 55 °C, −10 °C, −20 °C and −30 °C are 167.5 mA h g−1, 128.8 mA h g−1, 116.0 mA h g−1 and 100.2 mA h g−1, respectively. The excellent performance of material 2LFP·LVP@C/G at high rate and extreme temperature could be attributed to 3D electron conducting network formed in the samples when precursors were mixed properly, which may increases electron conductivity so as to enhance rate capability and cycle ability.
The cyclic voltammetry curves of the different composites at a scan rate of 0.1 mV s−1 between 2.5–4.5 V are presented in Fig. 6a. The cathodic and anodic peaks indicate the potential difference between the oxidation and reduction processes, which was ascribed to the lithium extraction and insertion, respectively. The oxidation curve in the 2LFP·LVP@C/G consist of 4 anodic peaks at approximately 3.519 V, 3.617 V, 3.695 V and 4.114 V, while the reduction curve include corresponding cathodic peaks at 3.339 V, 3.549 V, 3.627 V and 4.007 V. Compared with 2LFP·LVP@C, it is noticeable that 2LFP·LVP@C/G exhibits more symmetrical and shaper of peaks and the voltage gap between the redox peaks is obviously much smaller, which indicate that 2LFP·LVP@C/G presents much better reversibility for Li+ extraction/insertion. These results are in accordance with the charge and discharge curves obtained previously.
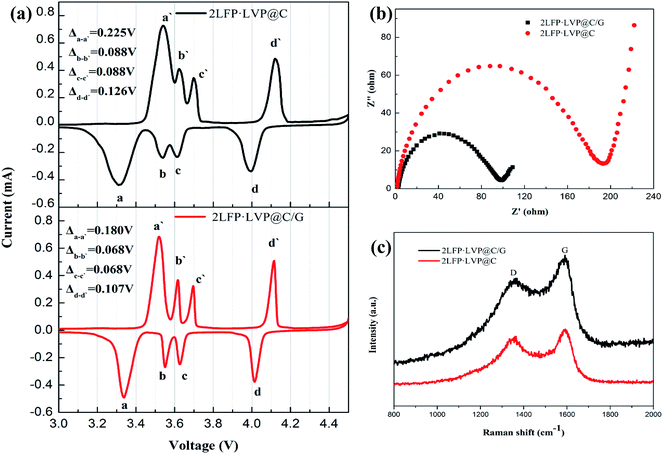 | ||
| Fig. 6 (a) Cyclic voltammetry profiles of the 2LFP·LVP@C/G and 2LFP·LVP@C. (b) Electrochemical impedance spectroscopy of 2LFP·LVP@C/G and 2LFP·LVP@C. (c) Raman spectra of 2LFP·LVP@C/G and 2LFP·LVP@C. | ||
Fig. 6b shows the electrochemical impedance spectroscopy of different composites after the first charge–discharge rate at 0.1 C. Each spectrum consists of a semicircle in the high-frequency region and a straight line in the low-frequency region. The depressed semicircle in high-frequency region was attributed to the charge-transfer resistance (Rct) of the electrochemical reaction while the straight line was related to the diffusion-controlled Warburg impedance. It clearly shows that 2LFP·LVP@C/G exhibits a smaller semicircle than that of 2LFP·LVP@C, which indicates that reduced graphene oxide reduces charge transfer resistance and thus improves the electronic conductivity.
Raman spectroscopy has been recognized as one of the most sensitive tools for studying the structural properties of carbonaceous materials, and the result of the 2LFP·LVP@C/G composite material is shown in Fig. 6c. Two broad bands were detected at around 1586 cm−1 and 1358 cm−1 in the spectrum, which are ascribed to D band and G band, respectively. As D band is a breathing mode of k-point phonons of A1g symmetry and G band is usually assigned to E2g phonon of C sp2 hybridized atoms,46 the sp3/sp2 bond quantity in material can be estimated by measuring the ratio of D and G band intensities ID/IG. This helps to evaluate the amount of graphene relative to other carbon materials, as it is known that carbon atoms in graphene are sp2 hybridized. Compared with the ID/IG ratio of 0.898 for 2LFP·LVP@C, the 2LFP·LVP@C/G displays a lower ratio of 0.827, indicating that the presence of graphene in 2LFP·LVP@C/G. Most of the studies suggest that lower ID/IG ratios provide higher cathode discharge capacity at high discharge rates.47–50
The excellent electrochemical performance of the composites may be due to the tightly contact between 2LFP·LVP@C and graphene. From the view of synergy effects, the resultant composite is not merely the sum of 2LFP·LVP@C and graphene, but rather a new material with new functionalities and properties. On the one hand, 2LFP·LVP@C anchored on grapheme suppress the agglomeration and restacking of graphene that increase the available surface area of the grapheme, accompanied with high electrochemical activity. On the other hand, as a support, graphene can induce nucleation, growth and formation of 2LFP·LVP@C, which is uniform dispersion and controlled morphology on the surface of graphene with high chemical functionality. Therefore, 2LFP·LVP@C/G can form an excellent integrated structure with a 3D electron conductive net work.
4 Conclusion
In summary, a novel 3D hierarchical 2LFP·LVP@C/G composite cathode were synthesized via conventional solid-state method with the home-made Fe4(VO4)4·15H2O/GO precursor. Compared with 2LFP·LVP@C, 2LFP·LVP@C/G is more favorable for electron migration because of synergy effects, which indicate predominant electrochemical performance. The discharge capacities of 2LFP·LVP@C/G at the rate of 0.1 C, 1 C, 2 C, 5 C and 10 C are 152.1 mA h g−1, 142.4 mA h g−1, 136.6 mA h g−1, 131.7 mA h g−1 and 126.2 mA h g−1, respectively, while the discharge capacities at 0.1 C with the temperature of 55 °C, −10 °C, −20 °C and −30 °C are 167.5 mA h g−1, 128.8 mA h g−1, 116.0 mA h g−1 and 100.2 mA h g−1, respectively. The present work demonstrates that graphene incorporation is an efficient approach for 2LiFePO4·Li3V2(PO4)3 to improve the electrochemical performance.Acknowledgements
We gratefully acknowledge the financial support for this work of the National Natural Science Foundation of China under grant number 51402365, and China Postdoctoral Science Foundation (2014M560651).References
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188–1194 CrossRef CAS PubMed.
- C. Delacourt, P. Poizot, M. Morcrette, J. M. Tarascon and C. Masquelier, Chem. Mater., 2004, 16, 93 CrossRef CAS.
- X. L. Wu, L. Y. Jiang, F. F. Cao, Y. G. Guo and L. J. Wan, Adv. Mater., 2009, 21, 2710 CrossRef CAS.
- M. M. Ren, Z. Zhou, Y. Z. Li, X. P. Gao and J. Yan, J. Power Sources, 2006, 162, 1357 CrossRef CAS PubMed.
- M. Y. Saidi, J. Barker, H. Huang, J. L. Sowyer and G. Adamson, J. Power Sources, 2003, 119–121, 266 CrossRef CAS.
- B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691–714 CrossRef CAS.
- S. Ferrari, R. L. Lavall, D. Capsoni, E. Quartarone, A. Magistris, P. Mustarelli and P. Canton, J. Phys. Chem. C, 2010, 114, 12598–12603 CAS.
- H. Huang, S. C. Yin, T. Kerr, N. Taylor and L. F. Nazar, Adv. Mater., 2002, 14, 1525 CrossRef CAS.
- S. C. Yin, H. Grondey, P. Strobel, H. Huang and L. F. Nazar, J. Am. Chem. Soc., 2003, 125, 326–327 CrossRef CAS PubMed.
- G. Yang, H. D. Liu, H. M. Ji, Z. Z. Chen and X. F. Jiang, Electrochim. Acta, 2010, 55, 2951–2957 CrossRef CAS PubMed.
- F. Omenya, N. A. Chernova, S. Upreti, P. Y. Zavalij, K. W. Nam, X. Q. Yang and M. S. Whittingham, Chem. Mater., 2011, 23, 4733–4740 CrossRef CAS.
- L. L. Zhang, G. Liang, A. Ignatov, M. C. Croft, X. Q. Xiong, I. M. Hung, Y. H. Huang, X. L. Hu, W. X. Zhang and Y. L. Peng, J. Phys. Chem. C, 2011, 115, 13520–13527 CAS.
- M. R. Yang, W. H. Ke and S. H. Wu, J. Power Sources, 2007, 165, 646–650 CrossRef CAS PubMed.
- L. N. Wang, Z. C. Li, H. J. Xu and K. L. Zhang, J. Phys. Chem. C, 2008, 112, 308–312 CAS.
- S. K. Zhong, L. Wu and J. Q. Liu, Electrochim. Acta, 2012, 74, 8–15 CrossRef CAS PubMed.
- Y. Guo, Y. D. Huang, D. Z. Jia, X. C. Wang, N. Sharma, Z. P. Guo and X. C. Tang, J. Power Sources, 2014, 246, 912–917 CrossRef CAS PubMed.
- J. F. Zhang, C. Shen, B. Zhang, J. C. Zheng, C. L. Peng, X. W. Wang, H. Li, X. B. Yuan and G. M. Chen, J. Power Sources, 2014, 267, 227–234 CrossRef CAS PubMed.
- H. Tang, X. D. Guo, B. H. Zhong, H. Liu, Y. Tang, R. Xu and L. Y. Li, J. Solid State Electrochem., 2012, 16, 1537–1543 CrossRef CAS PubMed.
- J. C. Zheng, X. H. Li, Z. X. Wang, J. H. Li, L. J. Li, L. Wu and H. J. Guo, Ionics, 2009, 15, 753–759 CrossRef CAS PubMed.
- X. J. Chen, G. S. Cao, X. B. Zhao, J. P. Tu and T. J. Zhu, J. Alloys Compd., 2008, 463, 385–389 CrossRef CAS PubMed.
- J. Y. Xiang, J. P. Tu, L. Zhang, X. L. Wang, Y. Zhou, Y. Q. Qiao and Y. Lu, J. Power Sources, 2010, 195, 8331–8335 CrossRef CAS PubMed.
- B. Zhang, J. C. Zheng and Z. H. Yang, Ionics, 2011, 17, 859–862 CrossRef CAS.
- S. K. Zhong, L. Wu, J. C. Zheng and J. Q. Liu, Powder Technol., 2012, 219, 45–48 CrossRef CAS PubMed.
- J. Hong, X. L. Wang, Q. Wang, F. Omenya, N. A. Chernova, M. S. Whittingham and J. Graetz, J. Phys. Chem. C, 2012, 116, 20787–20793 CAS.
- H. Lin, Y. W. Wen, C. X. Zhang, L. L. Zhang, Y. H. Huang, B. Shan and R. Chen, Solid State Commun., 2012, 152, 999–1003 CrossRef CAS PubMed.
- X. P. Zhang, H. J. Guo, X. H. Li, Z. X. Wang and L. Wu, Solid State Ionics, 2012, 212, 106–111 CrossRef CAS PubMed.
- N. Hua, C. Y. Wang, X. Y. Kang, T. Wumair and Y. Han, J. Alloys Compd., 2010, 503, 204–208 CrossRef CAS PubMed.
- G. Yang, C. Y. Jiang, X. M. He, J. R. Ying and F. P. Cai, Ionics, 2012, 18, 59–64 CrossRef CAS.
- J. C. Zheng, X. H. Li, Z. X. Wang, J. H. Li, L. Wu, L. J. Li and H. J. Guo, Acta Phys.-Chim. Sin., 2009, 25, 1916–1920 CAS.
- J. C. Zheng, X. H. Li, Z. X. Wang, J. H. Li, L. J. Li, L. Wu and H. J. Guo, Ionics, 2009, 15, 753–759 CrossRef CAS PubMed.
- G. Yang, C. Y. Jiang, X. M. He, J. R. Ying and J. Gao, Ionics, 2013, 19, 1247–1253 CrossRef CAS.
- F. Yu, L. Zhang, Y. Li, Y. An, M. Zhu and B. Dai, RSC Adv., 2014, 4, 54576–54602 RSC.
- F. Yu, P. QI, Y. An, G. Wang, L. Xia, M. Zhu and B. Dai, Energy Technol., 2015 DOI:10.1002/ente.201402160.
- M. R. Yang, W. H. Ke and S. H. Wu, J. Power Sources, 2007, 165, 646–650 CrossRef CAS PubMed.
- L. Wu, J. J. Lu and S. K. Zhong, J. Solid State Electrochem., 2013, 17, 2235–2241 CrossRef CAS PubMed.
- J. C. Zheng, X. H. Li, Z. X. Wang, D. M. Qin, H. J. Guo and W. J. Peng, J. Inorg. Mater., 2009, 24, 143–146 CrossRef CAS.
- Z. P. Ma, G. J. Shao, X. Wang, J. J. Song and G. L. Wang, Ionics, 2013, 19, 1861–1866 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- A. Peigney, C. Laurent, E. Flahaut, R. R. Bacsa and A. Rousset, Carbon, 2001, 39, 507–514 CrossRef CAS.
- G. Kucinskis, G. Bajars and J. Kleperis, J. Power Sources, 2013, 240, 66–79 CrossRef CAS PubMed.
- Y. Ding, Y. Jiang, F. Xu, J. Yin, H. Ren and Q. Zhuo, Electrochem. Commun., 2010, 12, 10–13 CrossRef CAS PubMed.
- X. F. Zhou, F. Wang, Y. M. Zhu and Z. P. Liu, J. Mater. Chem., 2011, 21, 3353–3358 RSC.
- H. D. Liu, P. Gao, J. H. Fang and G. Yang, Chem. Commun., 2011, 47, 9110 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- X. Y. Yang, X. Y. Zhang, Y. F. Ma, Y. Huang, Y. S. Wang and Y. S. Chen, J. Mater. Chem., 2009, 19, 271 Search PubMed.
- Z. Luo, T. Yu, J. Shang, Y. Wang, S. Lim, L. Liu, G. G. Gurzadyan, Z. Shen and J. Lin, Adv. Funct. Mater., 2011, 21, 911 CrossRef CAS.
- J. Yang, J. Wang, D. Wang, X. Li, D. Geng, G. Liang, M. Gauthier, R. Li and X. Sun, J. Power Sources, 2012, 208, 340 CrossRef CAS PubMed.
- X. Zhou, F. Wang, Y. Zhu and Z. Liu, J. Mater. Chem., 2011, 21, 3353 RSC.
- O. Toprakci, H. A. K. Toprakci, L. Ji, Z. Lin, R. Gu and X. Zhang, J. Renewable Sustainable Energy, 2012, 4, 013121 CrossRef PubMed.
- M. M. Doeff, J. D. Wilcox, R. Kostecki and G. Lau, J. Power Sources, 2006, 163, 180 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |