Perfluorobutyl iodide-assisted direct cyanomethylation of azoles and phenols with acetonitrile†
Juan Zhang,
Wei Wu,
Xinfei Ji and
Song Cao*
Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology (ECUST), Shanghai 200237, China. E-mail: scao@ecust.edu.cn; Fax: +86-21-64252603; Tel: +86-21-64253452
First published on 10th February 2015
Abstract
A perfluorobutyl iodide-assisted transition-metal-free cyanomethylation of azoles and phenols with acetonitrile in the presence of NaH has been developed. The reaction proceeded smoothly under mild reaction conditions to give the cyanomethylated products in moderate to high yields. A mechanism involving the cyanomethyl radical through C–H bond cleavage in acetonitrile was proposed.
The cyanomethylation of organic compounds is an important chemical transformation due to the wide utility of the nitrile group as a potent synthetic block for the construction of diverse complex molecules.1 Recently, extensive efforts have been made toward the development of methods for introducing the cyanomethyl group to different substrates.2 As a consequence, various novel cyanomethylation reactions have increasingly emerged such as the photoassisted direct cyanomethylation of benzene with acetonitrile in the presence of Pd/TiO2,3 the cyanomethylation of aromatic alcohols with acetonitrile using CuCl2 as the catalyst and O2 as the oxidant4 and the nickel cyanomethyl complex-catalyzed the coupling of aldehydes and acetonitrile.5
The methods for the preparation of cyanomethylated compounds typically require the use of prefunctionalized acetonitrile, including haloacetonitrile,6 Me3SiCH2CN (TMSAN)7 and isoxazole-4-boronic acid pinacol ester (acetonitrile anion equivalent).8 The direct use of acetonitrile as starting material to undergo cyanomethylation reaction has attracted much interest in organic chemistry since it avoids substrate prefunctionalization.9 Traditionally, the deprotonation of acetonitrile could proceed smoothly in the presence of strong bases (such as sodium amide) at very low temperature,10 but the harsh reaction conditions limit this method.
Nowadays, transition-metal-catalyzed C–H bond activation of acetonitrile has become a straightforward and viable alternative approach to cyanomethylated compounds.11 To date, most of the research in this area focuses on the cyanomethylation of carbonyl compounds,12 activated alkenes (N-phenylacrylamides)13 and imines.14 However, direct N- and O-cyanomethylation of azole and phenol with acetonitrile still remains challenging.
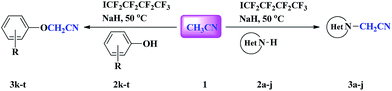
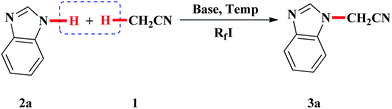
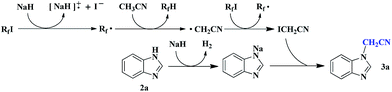
In addition, perfluoroalkyl iodides are usually utilized as perfluoroalkyl radical precursors.15 However, the formation of radicals from these perfluoroalkyl radical sources is often initiated by precious metal photocatalysts,16 radical initiators,17 peroxides,18 heat and light irradiation.19 In 2014, Zhang and Studer reported the Rf–I bond in perfluoroalkyl iodide could be homolytically cleaved to generate a perfluoroalkyl radical with the assistance of Cs2CO3 in the absence of metal salt initiator.20 Herein, we report the first example of NaH-mediated direct N- and O-cyanomethylation of azole and phenol via perfluorobutyl iodide-assisted cleavage of C–H bond of acetonitrile without transition metal catalyst (Scheme 1).
At the start of our investigations, we chose the reaction between benzimidazole 2a and acetonitrile in the presence of perfluorobutyl iodide as a model reaction to survey the reaction conditions (Table 1). Among the bases tested, NaH proved to be the most effective base for this reaction, affording product 3a in 90% yield (entry 7). The use of other bases led to decreased yields (entries 1–6). No detectable amount of N-cyanomethylated benzimidazole 3a was formed in the absence of base (entry 8). It was found that three equivalents of NaH were necessary to achieve this transformation (entries 7 and 9–11), but if the amount of NaH was increased to 3.5 equiv., the yield of 3a decreased obviously (entry 12).
| Entry | CH3CN (mL) | RfI (equiv.) | Temp (°C) | Base (equiv.) | Yield of 3ab(%) |
|---|---|---|---|---|---|
| a Reaction conditions: 2a (0.25 mmol), 24 h.b Yields determined by GC analysis and based on 2a. | |||||
| 1 | 2.5 | C4F9I (1.2) | 50 | NaOH (3.0) | 10 |
| 2 | 2.5 | C4F9I (1.2) | 50 | KOH (3.0) | 25 |
| 3 | 2.5 | C4F9I (1.2) | 50 | K2CO3 (3.0) | 45 |
| 4 | 2.5 | C4F9I (1.2) | 50 | K3PO4 (3.0) | 50 |
| 5 | 2.5 | C4F9I (1.2) | 50 | tBuOK (3.0) | 66 |
| 6 | 2.5 | C4F9I (1.2) | 50 | Cs2CO3 (3.0) | 70 |
| 7 | 2.5 | C4F9I (1.2) | 50 | NaH (3.0) | 90 |
| 8 | 2.5 | C4F9I (1.2) | 50 | None | 0 |
| 9 | 2.5 | C4F9I (1.2) | 50 | NaH (1.5) | 70 |
| 10 | 2.5 | C4F9I (1.2) | 50 | NaH (2.0) | 83 |
| 11 | 2.5 | C4F9I (1.2) | 50 | NaH (2.5) | 85 |
| 12 | 2.5 | C4F9I (1.2) | 50 | NaH (3.5) | 74 |
| 13 | 2.5 | C4F9I (0.5) | 50 | NaH (3.0) | 50 |
| 14 | 2.5 | C4F9I (1.0) | 50 | NaH (3.0) | 76 |
| 15 | 2.5 | C4F9I (1.5) | 50 | NaH (3.0) | 88 |
| 16 | 2.5 | None | 50 | NaH (3.0) | 0 |
| 17 | 1.5 | C4F9I (1.2) | 50 | NaH (3.0) | 73 |
| 18 | 3.5 | C4F9I (1.2) | 50 | NaH (3.0) | 85 |
| 19 | 4.0 | C4F9I (1.2) | 50 | NaH (3.0) | 83 |
| 20 | 2.5 | C4F9I (1.2) | 25 | NaH (3.0) | 70 |
| 21 | 2.5 | C4F9I (1.2) | 65 | NaH (3.0) | 45 |
| 22 | 2.5 | CF3(CF2)7I (1.2) | 50 | NaH (3.0) | 85 |
The addition of 1.2 equiv. of perfluorobutyl iodide was required to obtain satisfactory yield (entry 7 and entries 13–15). The reaction did not proceed in the absence of perfluorobutyl iodide (entry 16). The acetonitrile was used as both substrate and solvent. Increasing or decreasing the amount of CH3CN slightly diminished the yield of 3a (entries 17–19). The use of 2.5 mL of CH3CN could provide a good yield. The effect of the temperature on the reaction was also examined (entries 20–21). The results indicated that 50 °C was the optimal temperature for this reaction. Finally, no obvious difference was observed by replacing perfluorobutyl iodide with perfluorooctyl iodide (entry 22).
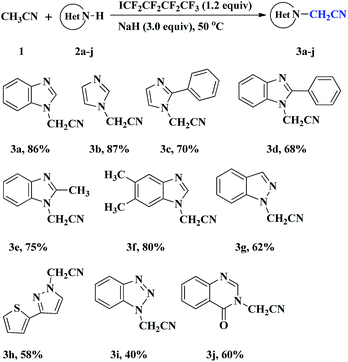
To survey the generality of this transformation, a variety of azoles were allowed to react with acetonitrile under the optimized conditions as in entry 7 in Table 1. The results are summarized in Table 2. Most azoles could afford the N-cyanomethylated products in moderate to high yields. Both benzoimidazole derivatives (2a, 2d, 2e and 2f) and imidazole derivatives (2b and 2c) underwent the cyanomethylation reaction smoothly irrespective of whether benzene ring is present or not. The optimized conditions were also suitable for the N-cyanomethylation of benzo-fused pyrazole (1H-indazole, 2g) and pyrazole derivative 2h, however, the yields decreased appreciably. 1H-Benzo[d][1,2,3]triazole 2i could react with acetonitrile, but the desired product 3i was obtained in low yield. It is noteworthy that quinazolin-4(3H)-one 2j is also compatible with this reaction and the cyanomethylated product was obtained in moderate yield. Unfortunately, when 1H-indole was used as substrate, less than 20% of the expected product was detected (GC-MS). It appears that the basicity of the azoles played a crucial role in the reaction. With a decrease in the basicity, benzoimidazole or imidazole derivatives, 1H-indazole or 1H-pyrazole, 1H-benzo[d][1,2,3]triazole and 1H-indole led to sharply descending reaction efficiency. The order of reactivity, imidazole > pyrazole > 1H-benzo[d][1,2,3]triazole > 1H-indole, is in accordance with the order of their basicities.
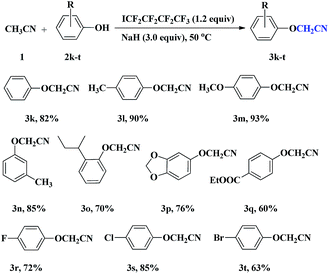
To expand the scope of this novel transformation, the reactions of acetonitrile 1 with various substituted phenols 2k–t were carried out under identical reaction conditions as outlined above and representative results were summarized in Table 3. Phenol derivatives with electron donating group such as CH3 and OCH3 (2l and 2m) at para position afforded excellent yields of aryloxyacetonitriles, whereas those with electron donating group at meta or ortho position provided the corresponding cyanomethylated products in moderate to good yields (3n–p). Phenols bearing relatively weak electron-withdrawing substituents such as ester and halogen were also tolerated, providing the products in moderate yields (3q–t). The reactions of 4-nitrophenol and 4-hydroxybenzonitrile with acetonitrile hardly proceeded and no desired products were observed due to the presence of strong electron-withdrawing group on the benzene ring.
In addition, when aniline, amino pyridine or benzyl alcohol was used as substrate, only trace amount of cyanomethylation product was detected. Furthermore, the replacement of acetonitrile with propionitrile or phenylacetonitrile led to the failure of this novel reaction. It might be due to steric effect of ethyl and phenyl group.
To gain possible insights into the reaction mechanism, several control experiments were conducted. Under the optimized reaction conditions, a radical scavenger such as hydroquinone (0.5 equiv.), TEMPO (2.0 equiv.) and galvinoxyl (2.0 equiv.) was added separately, the yields of the reactions between benzimidazole 2a and acetonitrile 1 decreased significantly (7%, 56% and 35%, respectively). These studies indicated that the reaction may proceed via a radical pathway. In addition, when the reaction was performed in the absence of benzimidazole 2a in the model reaction, 2-iodoacetonitrile was detected (19%, GC-MS). This result clearly revealed that the reaction involved the formation of the key intermediate ICH2CN in the earlier stage of the reaction.
Based on the above observations, a plausible reaction mechanism is depicted in Scheme 2 (with 2a as the example). It has been reported that Cs2CO3,20,21a Na2CO3,21b enamines21c,d could be used as a radical initiator for the cleavage of C–I bond in perfluoroalkyl iodide to produce perfluoroalkyl radical (R˙f). In the course of optimization of the reaction conditions, we found that other base such as Cs2CO3, tBuOK, K3PO4 and K2CO3 could also provide moderate yield of desired product. Therefore, we assume that NaH worked as radical initiator.22 In initiation step, the cleavage of C–I bond in perfluoroalkyl iodide with the assistance of NaH resulted in the formation of perfluoroalkyl radical (R˙f). The Rf radical can undergo hydrogen abstraction from the CH3CN to generate the cyanomethyl radical (˙CH2CN) and RfH. Subsequently, the cyanomethyl radical attacks perfluoroalkyl iodide to produce the key intermediate, ICH2CN along with perfluoroalkyl radical. Finally, the reaction of ICH2CN with sodium salt of benzimidazole afforded the cyanomethylated product 3a.
In summary, we have developed the first perfluoroalkyl iodide-promoted cyanomethylation of azoles and phenols with acetonitrile in the presence of NaH through a cyanomethyl radical pathway. The transformation proceeded efficiently in the absence of transition metal catalyst or other additional radical initiator. The main advantage of this method is that the acetonitrile could be used directly without prefunctionalization of it with halogen. Further studies to expand the scope of the C–H bond cleavage of CH3CN with the assistance of perfluorobutyl iodide are underway in our laboratory.
Acknowledgements
We are grateful for financial supports from the National Natural Science Foundation of China (Grant nos 21472043, 21272070), and the Key Project in the National Science & Technology Pillar Program of China in the twelfth five-year plan period (2011BAE06B01-15).Notes and references
- (a) G.-P. Yu, D. Kuo, M. Shoham and R. Viswanathan, ACS Comb. Sci., 2014, 16, 85 CrossRef CAS PubMed; (b) F.-R. Wu, H.-J. Zhu, L.-L. Sun, C. Rajendran, M.-T. Wang, X. Ren, S. Panjikar, A. Cherkasov, H.-B. Zou and J. Stöckigt, J. Am. Chem. Soc., 2012, 134, 1498 CrossRef CAS PubMed; (c) D. M. Drab, J. L. Shamshina, M. Smiglak, C. C. Hines, D. B. Cordes and R. D. Rogers, Chem. Commun., 2010, 46, 3544 RSC; (d) F. C. Schrader, S. Glinca, J. M. Sattler, H.-M. Dahse, G. A. Afanador, S. T. Prigge, M. Lanzer, A.-K. Mueller, G. Klebe and M. Schlitzer, ChemMedChem, 2013, 8, 442 CrossRef CAS PubMed.
- (a) L.-Y. Wu and J. F. Hartwig, J. Am. Chem. Soc., 2005, 127, 15824 CrossRef CAS PubMed; (b) Y. Satoh and Y. Obora, RSC Adv., 2014, 4, 15736 RSC; (c) F. Stazi, W. Maton, D. Castoldi, P. Westerduin, O. Curcuruto and S. Bacchi, Synthesis, 2010, 3332 CAS; (d) D. A. Culkin and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 9330 CrossRef CAS PubMed; (e) S. P. Khanapure and E. R. Biehl, J. Org. Chem., 1990, 55, 1471 CrossRef CAS.
- H. Yoshida, Y. Fujimura, H. Yuzawa, J. Kumagai and T. Yoshida, Chem. Commun., 2013, 49, 3793 RSC.
- J.-X. Shen, D.-J. Yang, Y.-X. Liu, S.-S. Qin, J.-W. Zhang, J.-K. Sun, C.-H. Liu, C.-Y. Liu, X.-M. Zhao, C.-H. Chu and R.-H. Liu, Org. Lett., 2014, 16, 350 CrossRef CAS PubMed.
- S. Chakraborty, Y. J. Patel, J. A. Krause and H.-R. Guan, Angew. Chem., Int. Ed., 2013, 52, 7523 CrossRef CAS PubMed.
- (a) A. Nortcliffe, N. P. Botting and D. O' Hagan, Org. Biomol. Chem., 2013, 11, 4657 RSC; (b) O. M. Ali, A. E.-G. E. Amr and E. E. Mostafa, Res. Chem. Intermed., 2014, 40, 1545 CrossRef CAS PubMed.
- (a) C. Verrier, S. Oudeyer, I. Dez and V. Levacher, Tetrahedron Lett., 2012, 53, 1958 CrossRef CAS PubMed; (b) Y.-C. Fan, G.-F. Du, W.-F. Sun, W. Kang and L. He, Tetrahedron Lett., 2012, 53, 2231 CrossRef CAS PubMed; (c) T. Mukaiyama and M. Michida, Chem. Lett., 2007, 36, 1244 CrossRef CAS; (d) F. Diaba, C. L. Houerou, M. Grignon-Dubois and P. Gerval, J. Org. Chem., 2000, 65, 907 CrossRef CAS.
- J. Velcicky, A. Soicke, R. Steiner and H.-G. Schmalz, J. Am. Chem. Soc., 2011, 133, 6948 CrossRef CAS PubMed.
- (a) T. Yamashita, J. Org. Chem., 1996, 61, 6438 CrossRef CAS; (b) M. Masui, K. Yamagata, C. Ueda and H. Ohmori, J. Chem. Soc., Chem. Commun., 1985, 272 RSC; (c) P. Kisanga, D. McLeod, B. D' Sa and J. Verkade, J. Org. Chem., 1999, 64, 3090 CrossRef CAS PubMed; (d) B. Batanero, C. M. Sánchez-Sánchez, V. Montiel, A. Aldaz and F. Barba, Electrochem. Commun., 2003, 5, 349 CrossRef CAS.
- (a) N.-S. Li, S. Yu and G. W. Kabalka, J. Org. Chem., 1995, 60, 5973 CrossRef CAS; (b) S.-J. Chang and T. L. Stuk, Synth. Commun., 2000, 30, 955 CrossRef CAS.
- (a) C.-D. Pan, H.-L. Zhang and C.-J. Zhu, Org. Biomol. Chem., 2015, 13, 361 RSC; (b) T. Deng, H.-J. Wang and C. Cai, Eur. J. Org. Chem., 2014, 7259 CrossRef CAS; (c) T. Wu, X. Mu and G.-S. Liu, Angew. Chem., Int. Ed., 2011, 50, 12578 CrossRef CAS PubMed.
- (a) G.-W. Wang, A.-X. Zhou, J.-J. Wang, R.-B. Hu and S.-D. Yang, Org. Lett., 2013, 15, 5270 CrossRef CAS PubMed; (b) N. Kumagai, S. Matsunaga and M. Shibasaki, J. Am. Chem. Soc., 2004, 126, 13632 CrossRef CAS PubMed; (c) E. Y. Ko, C. H. Lim and K.-H. Chung, Bull. Korean Chem. Soc., 2006, 27, 432 CrossRef CAS; (d) Y. Suto, N. Kumagai, S. Matsunaga, M. Kanai and M. Shibasaki, Org. Lett., 2003, 5, 3147 CrossRef CAS PubMed.
- J. Li, Z.-G. Wang, N.-J. Wu, G. Gao and J.-S. You, Chem. Commun., 2014, 50, 15049 RSC.
- T. Poisson, V. Gembus, S. Oudeyer, F. Marsais and V. Levacher, J. Org. Chem., 2009, 74, 3516 CrossRef CAS PubMed.
- (a) Q.-Q. Qi, Q.-L. Shen and L. Lu, J. Am. Chem. Soc., 2012, 134, 6548 CrossRef CAS PubMed; (b) C. Pooput, W. R. Dolbier Jr and M. Médebielle, J. Org. Chem., 2006, 71, 3564 CrossRef CAS PubMed; (c) E. Yoshioka, S. Kohtani, K. Sawai, Kentefu, E. Tanaka and H. Miyabe, J. Org. Chem., 2012, 77, 8588 CrossRef CAS PubMed.
- Y.-D. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef CAS PubMed.
- (a) M. Matsugi, M. Hasegawa, S. Hasebe, S. Takai, R. Suyama, Y. Wakita, K. Kudo, H. Imamura, T. Hayashi and S. Haga, Tetrahedron Lett., 2008, 49, 4189 CrossRef CAS PubMed; (b) V. Církva, B. Améduri, B. Boutevin, J. Kvíčala and O. Paleta, J. Fluorine Chem., 1995, 74, 97 CrossRef.
- A. Bravo, H.-R. Bjørsvik, F. Fontana, L. Liguori, A. Mele and F. Minisci, J. Org. Chem., 1997, 62, 7128 CrossRef CAS PubMed.
- (a) T. Yajima, I. Jahan, T. Tonoi, M. Shinmen, A. Nishikawa, K. Yamaguchi, I. Sekine and H. Nagano, Tetrahedron, 2012, 68, 6856 CrossRef CAS PubMed; (b) S. Barata-Vallejo, M. M. Flesia, B. Lantaño, J. E. Argüello, A. B. Peñéñory and A. Postigo, Eur. J. Org. Chem., 2013, 998 CrossRef CAS.
- B. Zhang and A. Studer, Org. Lett., 2014, 16, 3990 CrossRef CAS PubMed.
- (a) T. Xu, C. W. Cheung and X.-L. Hu, Angew. Chem., Int. Ed., 2014, 53, 4910 CrossRef CAS PubMed; (b) Y. Aihara, M. Tobisu, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2014, 136, 15509 CrossRef CAS PubMed; (c) N. O. Brace, J. Org. Chem., 1979, 44, 212 CrossRef CAS; (d) D. Cantacuzène, C. Wakselman and R. Dorme, J. Chem. Soc., Perkin Trans. 1, 1977, 1365 RSC.
- K. Narasaka and M. Kitamura, ARKIVOC, 2006, vii, 245 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02242h |
| This journal is © The Royal Society of Chemistry 2015 |