Brønsted acid-catalysed intramolecular ring opening of 2-(aryloxymethyl)-3-aryloxiranes leading to trans-4-arylchroman-3-ols: scope and limitations†
Abstract
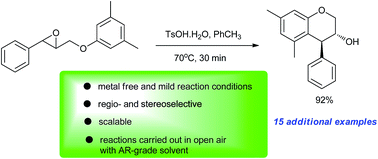
An operationally simple and metal-free method for the synthesis of a series of trans-4-arylchroman-3-ols via Brønsted acid (TsOH·H2O) catalysed stereoselective intramolecular Friedel–Crafts alkylation of electron-rich arenes by tethered epoxides is developed. The reactions neither need strict anhydrous conditions nor suffer from the use of expensive Lewis/Brønsted acids.

 Please wait while we load your content...
Please wait while we load your content...