Fabrication of SERS-active conjugated copolymers/gold nanoparticles composite films by interface-directed assembly†
Abstract
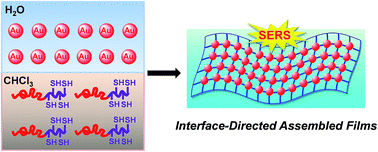
We report a new type of functional composite films by taking advantage of the interface-directed assembly between thiol groups functionalized poly(4-isocyano benzoic acid⋯pyridine-4-thiol)-b-poly(3-hexylthiophene) (PPI(–SH)-b-P3HT) conjugated copolymers and gold nanoparticles (Au NPs) at the chloroform/water interface. The PPI(–SH)-b-P3HT copolymers were synthesized through hydrogen bonding induced micellization and subsequent thiol–disulfide exchange reaction. Transmission electron microscopic (TEM) and atomic force microscopy (AFM) observations showed the film was uniform on a large scale and the integrity of surface morphology was not affected by the Au NPs concentration. Interestingly, the film substrate not only exhibited a strongly Au NPs concentration dependent surface-enhanced Raman scattering (SERS) activity but also allowed detection of model molecule, IR-792 perchlorate (IR-792), in the SERS measurement. This proof-of-concept suggests the interfacial assembly route is effective in integrating the properties of organic polymers and inorganic nanoparticles, and for further application.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...