Experimental and theoretical study of antioxidative properties of some salicylaldehyde and vanillic Schiff bases†
Zorica D. Petrović*a,
Jelena Đorovićb,
Dušica Simijonovića,
Vladimir P. Petrovića and
Zoran Markovićbc
aFaculty of Science, University of Kragujevac, Department of Chemistry, RadojaDomanovića 12, 34000 Kragujevac, Republic of Serbia. E-mail: zorica@kg.ac.rs; Fax: +381 34335040
bBioengineering Research and Development Center, 34000 Kragujevac, Republic of Serbia
cDepartment of Chemical-Technological Sciences, State University of Novi Pazar, Vuka Karadžića bb, 36300 Novi Pazar, Republic of Serbia
First published on 24th February 2015
Abstract
The antioxidative capacity and structure–activity relationships of ten Schiff bases were investigated experimentally and theoretically. All compounds contain the aniline moiety, while the aldehyde part is either salicylaldehyde or vanillin. The DPPH assay was used to test the potential antioxidative activity of these compounds, and DFT study was used to investigate their electronic structures and provide insight into their structure–activity relationships. The effect of the position of the hydroxy, as well other groups present, on the antioxidative activity was examined. The possible radical scavenging mechanism was determined in polar (water and methanol), and nonpolar (benzene) solvents. Based on the experimental and computational results, compounds 7 and 8 exhibit the highest radical scavenging properties.
Introduction
Schiff bases are compounds that were first obtained in the condensation reactions of aromatic amines and aldehydes (1864).1 They are also known as imines or azomethines.2 A wide range of these attractive compounds, with the general formula RHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R1 (R and R1 can be alkyl, aryl, cycloalkyl or heterocyclic groups), have been synthesized to date. Schiff bases are of great importance in the field of coordination chemistry because they are able to form stable complexes with metal ions.3 The chemical and biological significance of Schiff bases can be attributed to the presence of a lone electron pair in the sp2 hybridized orbital of the nitrogen atom of the azomethine group.4 These imines are used in the fields of organic synthesis, chemical catalysis and analysis, medicine, pharmacy, as well as other new technologies.5 The antitumor, antiviral, antifungal and antibacterial properties of these compounds means they have found applications in medicine and pharmacy.6 Due to these biological properties, Schiff bases are used as basic materials for the synthesis of many drugs.7 It has also been reported that Schiff bases of salicylaldehydes show some antimicrobial activity.8
N–R1 (R and R1 can be alkyl, aryl, cycloalkyl or heterocyclic groups), have been synthesized to date. Schiff bases are of great importance in the field of coordination chemistry because they are able to form stable complexes with metal ions.3 The chemical and biological significance of Schiff bases can be attributed to the presence of a lone electron pair in the sp2 hybridized orbital of the nitrogen atom of the azomethine group.4 These imines are used in the fields of organic synthesis, chemical catalysis and analysis, medicine, pharmacy, as well as other new technologies.5 The antitumor, antiviral, antifungal and antibacterial properties of these compounds means they have found applications in medicine and pharmacy.6 Due to these biological properties, Schiff bases are used as basic materials for the synthesis of many drugs.7 It has also been reported that Schiff bases of salicylaldehydes show some antimicrobial activity.8
The ability to scavenge free radicals is a common feature of phenolic compounds. Antioxidative activity of phenolic Schiff bases (SB–OH) is directly related to their ability to release hydrogen atoms. A few different mechanisms of free radical scavenging are known: hydrogen atom transfer (HAT), single electron transfer followed by proton transfer (SET-PT), and sequential proton loss electron transfer (SPLET).9 All these mechanisms have the same net result, i.e. the formation of corresponding phenoxy radical.10
HAT mechanism is the only one which consists of one step in which hydrogen atom is transferred to free radical.11
| SB–OH → SBO˙ + H˙ | (1) |
SET-PT and SPLET mechanisms consist of two steps. In SET-PT mechanism, the first step is characterized by process in which one electron is lost and radical cation is created, whereas in the second step radical cation is deprotonated and corresponding radical is formed.9b,12
| SB–OH → SB–OH˙+ + e− | (2.1) |
| SB–OH˙+ → SB–O˙ + H+ | (2.2) |
In SPLET mechanism, the first step is deprotonation of parent molecule. In the second step the anion formed loses an electron and corresponding radical is formed.13
| SB–OH → SB–O− + H+ | (3.1) |
| SB–O− → SB–O˙ + e− | (3.2) |
These mechanisms are described by thermodynamical parameters: bond dissociation enthalpy (BDE) related to eqn (1), ionization potential (IP) related to eqn (2.1), proton dissociation enthalpy (PDE) related to eqn (2.2), proton affinity (PA) related to eqn (3.1), and electron transfer enthalpy (ETE) related to eqn (3.2).
It is known that some phenolic Schiff bases act as effective antioxidants and potential drugs that can prevent disease caused by free radical damage.14 However, the antioxidative activity of this class of polyfunctional compounds deserves further investigation. Also, further advance in analysis of their structure–activity relationship, particularly how the position of the hydroxy group effects the reactivity of these phenolic compounds towards radicals is needed. In this sense, we put under consideration some salicylaldehyde and vanillic Schiff bases using experimental and theoretical tools.
The first part of this work is devoted to investigation of antioxidative capacity of ten phenolic Schiff bases, depending on substitution on the both phenyl rings – aldehyde and aniline. To fulfil this, DPPH assay is selected as method. Choice was made due to its well-known application in determination of the antioxidative activity of compounds,15 and due to fact that it can be used in prediction of activity against reactive oxygen species present in the living cells.16
Polarity of solvents plays significant role and specifies which mechanism to overcome. Bearing in mind this, further important aim of this paper was to estimate the solvent effects to the reaction enthalpies. To complete this, water and methanol were used as polar, whereas benzene was used as nonpolar solvent. To our best knowledge, this type of compounds, which can be considered as imine analogues of good antioxidant resveratrol, has not been subjected to this kind of study.
Results and discussion
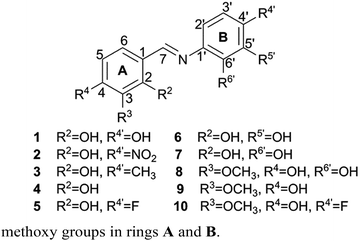
In the reaction of aldehyde (salicylaldehyde or vanillin) and aromatic amine (aniline, 4-fluoroaniline, 4-nitroaniline, toluidine, 2-hydroxyaniline, 3-hydroxyaniline or 4-hydroxyaniline) in methanol, a series of ten Schiff bases was synthesized (1–10, Fig. 1), wherein compound 10 is newly synthesized. The selection of these compounds was based on their structural characteristics, such as positions of hydroxy and methoxy groups in rings A and B.DPPH test
All of the obtained compounds were subjected to evaluation of their antioxidative activity in DPPH test, Tables 1 and S1 ESI.† It was found that compounds 2–6 are poor radical scavengers, while 1, 9, and 10 turned to be more active. Schiff bases 7 and 8 interact well with DPPH radical and exhibit high, slightly lower, activity than the reference compound NDGA. On the basis of this, compounds 7 and 8 can be considered as good antioxidants.| Compound | HOMO (eV) | LUMO (eV) | HOMO–LUMO gap (eV) | ΔEiso (kJ mol−1) | IC50 (μM) | ||
|---|---|---|---|---|---|---|---|
| a IC50 values obtained in this study. | |||||||
| Methanol | |||||||
| 1 | −0.274 | −0.039 | 0.234 | −13.038 | 561.3 (ref. 17) | 117.4 (ref. 18) | 186.3a |
| 2 | −0.293 | −0.075 | 0.218 | 41.638 | >500a | ||
| 3 | −0.280 | −0.040 | 0.239 | 42.042 | >500a | ||
| 4 | −0.285 | −0.041 | 0.244 | 43.145 | >500a | ||
| 5 | −0.285 | −0.042 | 0.243 | 42.827 | >500a | ||
| 6 | −0.283 | −0.043 | 0.240 | 1.896 | 468.2 (ref. 17) | 406.9 (ref. 18) | >500a |
| 7 | −0.280 | −0.046 | 0.234 | −3.678 | 27.4 (ref. 17) | 98.5 (ref. 18) | 18.8a |
| 8 | −0.269 | −0.041 | 0.227 | −11.227 | 5.3a | ||
| 9 | −0.273 | −0.033 | 0.240 | −13.385 | 86.2a | ||
| 10 | −0.273 | −0.034 | 0.239 | −13.188 | 68.8a | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Water | |||||||
| 1 | −0.274 | −0.040 | 0.234 | −13.015 | |||
| 2 | −0.293 | −0.075 | 0.218 | 41.310 | |||
| 3 | −0.280 | −0.041 | 0.239 | 41.656 | |||
| 4 | −0.285 | −0.042 | 0.244 | 42.775 | |||
| 5 | −0.285 | −0.042 | 0.243 | 42.486 | |||
| 6 | −0.283 | −0.043 | 0.240 | 1.961 | |||
| 7 | −0.280 | −0.046 | 0.234 | −3.946 | |||
| 8 | −0.269 | −0.041 | 0.227 | −11.479 | |||
| 9 | −0.273 | −0.033 | 0.240 | −13.592 | |||
| 10 | −0.273 | −0.034 | 0.239 | −13.422 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Benzene | |||||||
| 1 | −0.272 | −0.037 | 0.235 | −13.301 | |||
| 2 | −0.295 | −0.073 | 0.221 | 48.283 | |||
| 3 | −0.278 | −0.038 | 0.239 | 49.522 | |||
| 4 | −0.283 | −0.040 | 0.243 | 50.509 | |||
| 5 | −0.284 | −0.042 | 0.242 | 49.611 | |||
| 6 | −0.281 | −0.042 | 0.240 | 1.050 | |||
| 7 | −0.279 | −0.047 | 0.232 | 1.124 | |||
| 8 | −0.266 | −0.040 | 0.226 | −6.238 | |||
| 9 | −0.270 | −0.029 | 0.240 | −9.005 | |||
| 10 | −0.271 | −0.032 | 0.239 | −8.325 | |||
Obtained results in this study suggest that position of the hydroxy groups in the Schiff bases plays decisive role in the antioxidative activity. Namely, the active compounds possess p-hydroxy group in the ring A (8–10), or o-hydroxy group in the ring B (7 and 8), relatively to the positions 1 and 1′, Fig. 1. Less active compounds (2–5) are hydroxy substituted only in o-position of the ring A, while compound 6 bears additional hydroxy group in m-position of the ring B. The only difference between compounds 1 and 7 is substitution in the ring B, p- and o-respectively. On the basis of the IC50 values, it can be concluded that o-hydroxy position in the ring B is responsible for radical scavenging of these compounds. Common for the Schiff bases 8–10 is that hydroxy group is present in p- and methoxy group in m-position in the ring A. Taking into account IC50 for these molecules, as well as for the compound 1, one can conclude that presence of p-hydroxy group in the ring A contributes more to antiradical activity than equivalent substitution in the ring B. Observation that appearance of hydroxy group in the p-position of the ring A, as well as in o-position of the ring B contributes to the highest extent to the radical scavenging activity is additionally supported by the fact that the most active compound 8 possess hydroxy groups in both positions. We note in passing that substitution of the ring B by electron donor or acceptor functional groups, had negligible impact on the antioxidative activity.
The low activity of compounds 2–5 towards radical scavenging activity can be rationalised on the basis that only present hydroxy group in o-position in the ring A can form intramolecular hydrogen bond with nitrogen, and thus will be prevented to interact with DPPH. Although Schiff base 6, in addition to the hydroxy group in o-position in the ring A, has another one in the m-position in the ring B, it is reasonable to expect that radical obtained in m-position will not be stabilised by delocalisation of its unpaired electron over the entire molecule, but only over ring B. On the other hand, in active compounds, this stabilisation through delocalisation over both rings is possible.
Performed DPPH test provided insight into potential antioxidative activity of the investigated compounds. However, to obtain full insight in the structure–activity relationship of the investigated Schiff bases, further investigation on the electronic structure of these compounds was necessary. For the sake of completeness, DFT study of the compounds subjected to this examination was performed.
Density functional theory
All geometrical and conformational isomers of the investigated Schiff bases were determined, and their energies calculated. The most stable isomers of all compounds are presented in Fig. S1 of ESI.†To verify the quality of structures predicted by theoretical calculations, available crystal structures (those for compounds 1 and 7)19 were compared to their optimized structures (Tables S2 and S3,† respectively). As expected, the obtained geometrical parameters for all solvents used are in mutual, excellent agreement. Furthermore, the calculations reproduced the experimental bond lengths and angles, as well as dihedral angles very well. Some deviations between experimental and calculated structural characteristics are, certainly, a consequence of the fact that experimental values refer to the solid state, whereas calculated values refer to the solution. As the skeleton of other molecules under investigation is identical with these two, we can assume that their geometries are also well determined. Furthermore, assumption that Schiff bases 1–7 form hydrogen bond between hydroxy group in o-position of the ring A and nitrogen from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N is confirmed. On the basis of the lengths of these intramolecular hydrogen bonds (Tables S2 and S3†) they can be considered as strong.
N is confirmed. On the basis of the lengths of these intramolecular hydrogen bonds (Tables S2 and S3†) they can be considered as strong.
HOMO and LUMO
The HOMO and LUMO are delocalized through the entire molecule for all studied Schiff bases (Fig. S2†). The energies of HOMO and LUMO are very important parameter in defining the reactivity of molecules, because they usually take part in chemical reactions. The molecule which has the lower EHOMO has weak electron donating ability. On the other hand, the higher EHOMO implies that the molecule is a good electron donor.20 The EHOMO values for the Schiff bases are shown in Table 1. The molecules with hydroxy group in p-position and methoxy group in m-position in ring A show the largest EHOMO values of −0.269 eV for compound 8, and −0.273 eV for compounds 9 and 10. In contrast, molecules with hydroxy group in o-position in ring A (compounds 2–7) show decreased HOMO values. Consequently, these compounds have weaker electron donating ability than other Schiff bases. The results obtained in the present work indicate that the existence of structure that resembles vanillin in A-ring is important for the increased energy of HOMO orbitals, and thereby better antioxidative potential of these compounds. The o-OH group in ring B contributes to the increase of EHOMO, and cannot be neglected. All these results are in accordance with the experimental data for IC50 (Table 1).The HOMO–LUMO gap determines chemical reactivity. This energy is directly related to the easiness of excitation of investigated molecules. Data from Table 1 for HOMO–LUMO gap also suggest that 8 has the highest antioxidative potential since it has the lowest value.
Stabilization energies (ΔEiso)
Calculated ΔEiso values are presented in Table 1. On the basis of obtained values it is possible to find relative stability for the involved hydroxy and methoxy groups of investigated Schiff bases. Applying stabilization energy is a simple method to predict the antioxidative potential to scavenge free radicals. Obtained results in this study confirm the importance of p-hydroxy group in the ring A, and o-hydroxy group in the ring B, in the stabilization of radical species obtained after hydrogen atom abstraction. The presence of an additional methoxy group decreased ΔEiso for compounds 8–10, because of the fact that oxygen atoms can donate lone electron pairs to stabilize the corresponding semiquinone free radical.Bond dissociation enthalpy and proton affinity
Homolytic O–H bond cleavage of the investigated molecules yields corresponding radicals. The calculated BDE values are presented in Table 2. It is obvious that stability of the obtained semiquinone radicals plays a very important role in determining the antioxidative activity of Schiff bases. Distribution of spin density provides a reliable representation of reactivity and stability of free radicals.21 The radicals with good spin delocalization are formed more easily and they are more stable than those with localized spin density. SOMOs for the most stable radicals of investigated Schiff bases are presented in (Fig. 2) and distribution of the spin density for all radicals in methanol are presented in Fig. S3.† In all radicals formed by homolytic cleavage of the OH group in p-position, either in the rings A or B, spin density is delocalized over the involved oxygen, and o- and p-carbon atoms of the corresponding aromatic ring. Additional stabilization is achieved by delocalization across the double CN bond, as well as across o- and p-carbon atoms of the adjacent ring. As a consequence Schiff bases with hydroxy groups in p-position have the smallest BDE values (compounds 1, 8–10). Table 2 shows that BDE values of Schiff bases with hydroxy group in p-position are lower than those with hydroxy groups in m- and o-positions. The main reason for somewhat higher BDE values of m-hydroxy groups lies in the fact that less stable radicals are formed, and that there is less delocalization of spin density via CN double bond and another aromatic ring (6 in Fig. 2). As for hydroxy groups in o-position in the A ring, relatively strong intramolecular hydrogen bond with nitrogen is also responsible for significantly higher BDE values.| HAT | SET-PT | SPLET | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BDE | IP | PDE | PA | ETE | |||||
| A | B | A | B | A | B | A | B | ||
| Methanol | |||||||||
| 1 | 407 | 351 | 542 | 56 | 0 | 183 | 149 | 414 | 392 |
| 2 | 406 | 624 | −28 | 168 | 428 | ||||
| 3 | 406 | 559 | 38 | 181 | 416 | ||||
| 4 | 407 | 584 | 13 | 180 | 417 | ||||
| 5 | 407 | 586 | 11 | 179 | 419 | ||||
| 6 | 407 | 366 | 566 | 32 | −9 | 178 | 152 | 419 | 404 |
| 7 | 396 | 360 | 555 | 31 | −4 | 167 | 152 | 419 | 399 |
| 8 | 353 | 364 | 533 | 11 | 22 | 140 | 171 | 403 | 384 |
| 9 | 351 | 542 | 0 | 146 | 395 | ||||
| 10 | 351 | 541 | 0 | 146 | 396 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Water | |||||||||
| 1 | 412 | 356 | 522 | 71 | 16 | 191 | 159 | 401 | 379 |
| 2 | 411 | 603 | −12 | 177 | 415 | ||||
| 3 | 411 | 539 | 53 | 189 | 403 | ||||
| 4 | 412 | 564 | 29 | 189 | 404 | ||||
| 5 | 412 | 566 | 27 | 188 | 405 | ||||
| 6 | 412 | 371 | 546 | 47 | 7 | 187 | 161 | 406 | 391 |
| 7 | 401 | 365 | 535 | 47 | 11 | 176 | 161 | 406 | 386 |
| 8 | 358 | 369 | 513 | 26 | 37 | 150 | 179 | 389 | 371 |
| 9 | 356 | 522 | 15 | 155 | 381 | ||||
| 10 | 356 | 521 | 16 | 155 | 382 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Benzene | |||||||||
| 1 | 415 | 352 | 636 | 189 | 126 | 465 | 416 | 359 | 345 |
| 2 | 414 | 661 | 163 | 434 | 390 | ||||
| 3 | 415 | 650 | 174 | 464 | 360 | ||||
| 4 | 416 | 665 | 161 | 462 | 363 | ||||
| 5 | 415 | 639 | 186 | 457 | 368 | ||||
| 6 | 417 | 367 | 661 | 165 | 115 | 458 | 426 | 368 | 350 |
| 7 | 400 | 367 | 651 | 159 | 125 | 437 | 429 | 373 | 347 |
| 8 | 359 | 370 | 621 | 148 | 159 | 409 | 456 | 360 | 324 |
| 9 | 357 | 630 | 136 | 422 | 345 | ||||
| 10 | 357 | 633 | 134 | 418 | 349 | ||||
Heterolytic cleavage of the O–H bonds of Schiff bases leads to formation of the corresponding anions. Geometrical parameters for anions of Schiff bases 1 and 7 are listed in Tables S2 and S3.† In comparison to the parent molecules, there are significant changes in bond lengths in the ring where anion is formed, suggesting decrease of aromaticity of this ring. All anions, with corresponding charge distribution obtained by NBO analysis, are presented in Fig. S4.† The charge distribution shows that anions formed in the ring A are slightly better delocalized compared to those formed in the ring B. The decrease of negative charge on o- and p-oxygen atoms is a consequence of good delocalization of negative charge over the ring A and CN double bond, and across o- and p-carbon atoms of the adjacent ring (Fig. S3†).
Ionization potential
The ionization potential (IP) illustrates the easiness of electron donation of phenolic compounds. It is well known that molecules with lower IP values are more active. The obtained IP values of investigated compounds are presented in Table 2. Comparison of the IP values from Table 2 with IP values of other Schiff bases,22 showed that the values obtained in this study are generally somewhat lower. This is due to using different approaches for calculating these parameters. On the basis of our results, molecules with only one o-hydroxy group in the ring A (compounds 2–5) have generally higher IP values. Introducing another hydroxy group in the ring B in m-, o-, or p-position (compounds 6, 7, and 1), additionally reduces the IP value. This is consistent with the well-known fact that position of the hydroxy group in the molecule plays a very important role for the electron donating capacity. The IP values decrease in molecules with additional methoxy group in m-position (compounds 9 and 10). Moreover, introduction of another hydroxy group in ring B in o-position, additionally reduces the IP values (compound 8). This implies that this compound has increased electron donor capacity, which facilitates the formation of the radical cation.Antioxidative mechanisms
On the basis of values of thermodynamical parameters (BDE, IP, PDE, PA, and ETE) prevailing antioxidative mechanism of Schiff bases in a corresponding solvent can be predicted.23 The lowest value for some parameter points out which reaction mechanism is thermodynamically more probable. All thermodynamical parameters for the investigated Schiff bases were calculated using M05-2X/6-311+G(d,p) in water, methanol, and benzene (Table 2). The obtained results show that compounds 2–6 have significantly higher BDE, IP, and PA values than other compounds under investigation. The values of thermodynamical parameters undoubtedly show that negligible antioxidative activity can be expected for these compounds. These results are in agreement with experimental IC50 values Table 1. Mutual comparison of BDE, IP, and PA values in Table 2 for other compounds (1, 7–10) reveals that IP values are the largest, indicating that SET-PT mechanism is not a favourable reaction path in all investigated solvents.On the other hand, in polar solvents PA values are significantly lower than the corresponding BDEs. It means that the SPLET mechanism is a dominant reaction pathway in polar medium. Low PA values show that Schiff bases can easily undergo heterolytic dissociation of OH bonds and yield the corresponding phenoxide anions. Taking this into account, it is reasonable to expect that SPLET mechanism prevails under physiological conditions (pH of 7.4). BDE and PA values in Table 2 show that HAT and SPLET mechanisms are competitive in nonpolar solvent.
Conclusions
In this study, antioxidative capacity of some phenolic Schiff bases has been examined, using experimental and theoretical tools. Two of investigated compounds, one salicylaldehyde (7) and one vanillic (8) Schiff base, can be considered as good radical scavengers. p-Hydroxy group in ring A, as well as o-in the ring B, are responsible for the antioxidative activity of these compounds. Furthermore, DFT examination showed that presence hydroxy groups in respective positions influences the increase of EHOMO, lowers HOMO–LUMO gap, and in that way contributes to better antioxidative potential of these compounds. Low activity of Schiff bases 2–6 is assigned to the intramolecular hydrogen bond formation between o-hydroxy group in the ring A and nitrogen from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, which consequences significantly higher values for the thermodinamical parameters for these compounds. Based on the same data for the molecules which exhibit radical scavenging activity (1, 7–10), SET-PT mechanism is not expected in all investigated solvents. In polar medium, SPLET mechanism prevails, while HAT and SPLET mechanisms are competitive in nonpolar solvent. Taking into account that Schiff bases 7 and 8 interact well with DPPH radical, and fact that DPPH assay may indicate activity of compounds towards reactive oxygen species present in the living cells, these compounds can be considered as good antioxidants and will be further investigated in vitro/vivo, for example on the cancer cell lines.
N, which consequences significantly higher values for the thermodinamical parameters for these compounds. Based on the same data for the molecules which exhibit radical scavenging activity (1, 7–10), SET-PT mechanism is not expected in all investigated solvents. In polar medium, SPLET mechanism prevails, while HAT and SPLET mechanisms are competitive in nonpolar solvent. Taking into account that Schiff bases 7 and 8 interact well with DPPH radical, and fact that DPPH assay may indicate activity of compounds towards reactive oxygen species present in the living cells, these compounds can be considered as good antioxidants and will be further investigated in vitro/vivo, for example on the cancer cell lines.
Experimental
Materials and reagents
The compounds salicylaldehyde, vanillin, aniline, 4-fluoroaniline, 4-nitroaniline, toluidine, 2-hydroxyaniline, 3-hydroxyaniline, 4-hydroxyaniline, nordihydroguaeretic acid (NDGA), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Aldrich Chemical Co. All common chemicals were of reagent grade. The NMR spectra were run in DMSO on a Varian Gemini 200 MHz spectrometer. Melting points were determined on a Mel-Temp capillary melting points apparatus, model 1001. Elemental microanalyse for carbon, hydrogen, and nitrogen were performed at the Faculty of Chemistry, University of Belgrade.Synthesis of Schiff bases
Schiff bases (1–10) were prepared according to procedure in the literature with some modifications.24 In our case, aldehyde (salicylaldehyde, vanillin) (1 mmol), corresponding aromatic amine (aniline, 4-fluoroaniline, 4-nitroaniline, toluidine, 2-hydroxyaniline, 3-hydroxyaniline, 4-hydroxyaniline) (1 mmol), and 3 mL of methanol were placed in flask and stirred at 70 °C for 3 h. After completion of the reaction, the solvent was evaporated, and final product was obtained by recrystallization from ethanol. Schiff bases were obtained in 90–97% yield. All Schiff bases (1–10) were characterized with melting point and 1H NMR spectra (ESI,† compounds 1–10).The corresponding data for the new compound (E)-4-((4-fluorophenylimino)methyl)-2-methoxyphenol (10) are presented here: colourless crystals – mp 144–146 °C; 1H NMR (200 MHz, DMSO-d6): δ = 3.84 (s, 3H, −OCH3), 6.89 (d, J = 8.10 Hz, 1H, Ar-H), 7.16–7.28 (m, 5H, Ar-H), 7.32 (dd, J = 8.3, 1.9 Hz, 1H, Ar-H), 7.52 (d, J = 1.80 Hz, 1H, Ar-H), 8.44 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 9.77 (s, 3 –OH); 13C NMR (50 MHz, DMSO) δ = 56.7, 111.8, 111.4, 116.1, 116.5, 116.6, 117.2, 123.6, 127.5, 145.3, 149.21, 151.1, 153.9, 162.1; C14H12FNO2 (FW = 245.25): C, 68.56; N, 5.71; H, 4.93%; found: C, 68.15; N, 5.68; H, 4.81%.
N), 9.77 (s, 3 –OH); 13C NMR (50 MHz, DMSO) δ = 56.7, 111.8, 111.4, 116.1, 116.5, 116.6, 117.2, 123.6, 127.5, 145.3, 149.21, 151.1, 153.9, 162.1; C14H12FNO2 (FW = 245.25): C, 68.56; N, 5.71; H, 4.93%; found: C, 68.15; N, 5.68; H, 4.81%.
DPPH free radical scavenging assay
In this study, the free radical scavenging activity of the examined Schiff bases was performed using the DPPH method, according to ref. 25. In brief, 1 mL (0.1 mm) of DPPH solution in methanol was mixed with an equal volume of the tested compound (20 μL of compound solution in DMSO and 980 μL of methanol). The reaction mixture is left at room temperature for 30 and 60 min. After incubation the absorbance was measured at 517 nm. As control solution, methanol was used. IC50 values represent the concentration necessary to obtain 50% of a maximum scavenging capacity. NDGA was used as positive control with 96% activity at 0.1 mM.Computational details
All calculations in this paper were performed using the Gaussian program package.26 The equilibrium geometries of all Schiff bases and corresponding radicals, radical cations, and anions were optimized by the empirical exchange-correlation M05-2X functional27 and split-valence basis set 6-311+G(d,p). The M05-2X functional yields reasonable results for thermochemical calculations of organic, organometallic, and biological compounds, as well as for noncovalent interactions.11a,28 This functional has been successfully used for solution of different problems by independent authors.11b–eThe local and global minima were confirmed to be real minima by frequency analysis (no imaginary frequencies were obtained). To evaluate the impact of different solvents (water, methanol, and benzene) the continuum solvation model CPCM was used.29 The solvent effects were taken into account in all geometry optimizations and energy calculations. Water and benzene were used to mimic aqueous and lipid environments, whereas methanol was selected because the experiments were performed in this solvent. The NBO analysis of all species was performed at the M05-2X/6-311+G(d,p) level of theory.30 The NBO analysis describes a structure by a set of localized bonding, antibonding, and Rydberg orbitals. Also, this analysis provides explanation of stabilizing and destabilizing interactions between occupied and unoccupied orbitals.
BDE, IP, PDE, PA and ETE values were determined from total enthalpies of the individual species, as follows:
| BDE = H(SB–O˙) + H(H˙) − H(SB–OH) | (4) |
| IP = H(SB–OH˙+) + H(e−) − H(SB–OH) | (5) |
| PDE = H(SB–O˙) + H(H+) − H(SB–OH˙+) | (6) |
| PA = H(SB–O−) + H(H+) − H(SB–OH) | (7) |
| ETE = H(SB–O˙) + H(e−) − H(SB–O−) | (8) |
The values for solvation enthalpies of proton and electron were taken from literature.31 All reaction enthalpies defined in eqn (4)–(8) were calculated at 298 K.
The radical stability was determined by the calculation of stabilization energies (ΔEiso), as shown in eqn (9), where Ph–OH and Ph–O˙ stand for the molecule of phenol and phenoxy radical.
| ΔEiso = (H(SB–O˙) + H(Ph–OH)) − (H(SB–OH) + H(Ph–O˙)) | (9) |
Acknowledgements
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (projects no. 172016, 174028).Notes and references
- H. Schiff, Justus Liebigs Ann. Chem., 1864, 131, 118–119 CrossRef.
- S. Kalaivani, N. P. Priya and S. Arunachalam, Int. J. Appl. Biol. Pharm. Technol., 2012, 3, 219–223 CAS.
- P. Souza, J. A. Garcia-Vazquez and J. R. Masaguer, Transition Met. Chem., 1985, 10, 410–412 CrossRef CAS.
- (a) P. Singh, R. L. Goel and B. P. Singh, J. Indian Chem. Soc., 1975, 52, 958–959 CAS; (b) B. F. Perry, A. E. Beezer, R. J. Miles, B. W. Smith, J. Miller and M. G. Nascimento, Microbois., 1988, 45, 181–191 Search PubMed; (c) M. Kabak, A. Elmali and Y. Elerman, J. Mol. Struct., 1999, 477, 151–158 CrossRef CAS; (d) P. R. Patel, B. T. Thaker and S. Zele, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 1999, 38, 563–567 Search PubMed; (e) M. Valcarcel and M. D. Laque de Castro, Flow-Through Biochemical Sensors, Elsevier, Amsterdam, 1994 Search PubMed; (f) U. Spichiger-Keller, Chemical Sensors and Biosensors for Medical and Biological Applications, Wiley-VCH, Weinheim, 1998 Search PubMed; (g) J. F. Lawrence and R. W. Frei, Chemical Derivatization in Chromatography, Elsevier, Amsterdam, 1976 Search PubMed.
- (a) K. Tanaka, R. Shimoura and M. R. Caira, Tetrahedron Lett., 2010, 51, 449–452 CrossRef CAS PubMed; (b) A. S. Mocanu, M. Ilis, F. Dumitrascu, M. Ilie and V. Circu, Inorg. Chim. Acta, 2010, 363, 729–736 CrossRef CAS PubMed; (c) A. M. Atta, N. O. Shaker and N. E. Maysour, Prog. Org. Coat., 2006, 56, 100–110 CrossRef CAS PubMed; (d) J. H. Jia, X. M. Tao, Y. J. Li and W. J. Sheng, Chem. Phys. Lett., 2011, 514, 114–118 CrossRef CAS PubMed; (e) A. M. A. Ibrahim, Thermochim. Acta, 1992, 197, 211–217 CrossRef CAS.
- W. Radecka-Paryzek, I. Pospieszna-Markiewicz and M. Kubicki, Inorg. Chim. Acta, 2007, 360, 488–496 CrossRef CAS PubMed.
- (a) R. H. Lozier, R. A. Bogomolni and W. Stoeckenius, Biophys. J., 1975, 15, 955–962 CrossRef CAS; (b) E. M. Hodnett and W. J. Dunn, J. Med. Chem., 1970, 13, 768–770 CrossRef CAS.
- (a) C. Silva da, D. Silva da, L. Modolo and R. Alves, J. Adv. Res., 2011, 2, 1–8 CrossRef PubMed; (b) X. Yang, Q. Wang, Y. Huang, P. Fu, J. Zhang and R. Zeng, Inorg. Chem. Commun., 2012, 25, 55–59 CrossRef CAS PubMed; (c) K. Brodowska and E. Lodyga-Chruscinska, Chemik, 2014, 68, 129–134 CAS.
- (a) E. Klein, V. Lukeš and M. Ilčin, Chem. Phys., 2007, 336, 51–57 CrossRef CAS PubMed; (b) G. Litwinienko and K. U. Ingold, Acc. Chem. Res., 2007, 40, 222–230 CrossRef CAS PubMed.
- F. D. Meo, V. Lemaur, J. Cornil, R. Lazzaroni, J.-L. Duroux, Y. Olivier and P. Trouillas, J. Phys. Chem. A, 2013, 117, 2082–2092 CrossRef PubMed.
- (a) Y. Zhao, N. E. Schultz and D. G. Truhlar, J. Chem. Phys., 2005, 123, 161103 CrossRef PubMed; (b) M. E. Alberto, N. Russo, A. Grand and A. Galano, Phys. Chem. Chem. Phys., 2013, 15, 4642–4650 RSC; (c) G. Black and J. M. Simmie, J. Comput. Chem., 2010, 31, 1236–1248 CAS; (d) A. Galano and J. R. Alvarez-Idaboy, Org. Lett., 2009, 11, 5114–5117 CrossRef CAS PubMed; (e) A. Galano, N. A. Macias-Ruvalcaba, O. N. Medina-Campos and J. Pedraza-Chaverri, J. Phys. Chem. B, 2010, 114, 6625–6635 CrossRef CAS PubMed.
- Z. Marković, D. Amić, D. Milenković, J. M. Dimitrić Marković and S. Marković, Phys. Chem. Chem. Phys., 2013, 15, 7370–7378 RSC.
- (a) Z. S. Marković, J. M. Dimitrić Marković and Ć. B. Doličanin, Theor. Chem. Acc., 2010, 127, 69–80 CrossRef PubMed; (b) Z. Marković, D. Milenković, J. Đorović, J. M. Dimitrić Marković, V. Stepanić, B. Lučić and D. Amić, Food Chem., 2012, 135, 2070–2077 CrossRef PubMed; (c) C. Zavala-Oseguera, J. R. Alvarez-Idaboy, G. Merino and A. Galano, J. Phys. Chem. A, 2009, 113, 13913–13920 CrossRef CAS PubMed; (d) Z. Marković, D. Milenković, J. Đorović, J. M. Dimitrić Marković, V. Stepanić, B. Lučić and D. Amić, Food Chem., 2012, 134, 1754–1760 CrossRef PubMed.
- (a) L.-X. Chenga, J.-J. Tanga, H. Luob, X.-L. Jina, F. Daia, J. Yanga, Y.-P. Qiana, X.-Z. Lia and B. Zhoua, Bioorg. Med. Chem. Lett., 2010, 20, 2417–2420 CrossRef PubMed; (b) K. K. Upadhyay, A. Kumar, S. Upadhyay and P. C. Mishra, J. Mol. Struct., 2008, 873, 5–16 CrossRef CAS PubMed.
- M. Miliovskya, I. Svinyarov, Y. Mitrev, Y. Evstatieva, D. Nikolova, M. Chochkova and M. G. Bogdanov, Eur. J. Med. Chem., 2013, 66, 185–192 CrossRef PubMed.
- S.-Y. Li, X.-B. Wang and L.-Y. Kong, Eur. J. Med. Chem., 2014, 71, 36–45 CrossRef CAS PubMed.
- J. Lu, C. Li, Y.-F. Chai, D.-Y. Yang and C.-R. Sun, Bioorg. Med. Chem. Lett., 2012, 22, 5744–5747 CrossRef CAS PubMed.
- Y. Zhang, B. Zou, K. Wang, Y. Pan, H. Liang, X. Yi and H. Wang, Med. Chem. Res., 2012, 21, 1341–1346 CrossRef CAS.
- (a) Y. Wang, Z. Yua, Y. Sun, Y. Wang and L. Lua, Spectrochim. Acta, Part A, 2011, 79, 1475–1482 CrossRef CAS PubMed; (b) D. Maciejewska, D. Pawlak and V. Koleva, J. Phys. Org. Chem., 1999, 12, 875–880 CrossRef CAS.
- S. Antonczak, J. Mol. Struct., 2008, 856, 38–45 CrossRef CAS PubMed.
- (a) M. Leopoldini, T. Marino, N. Russo and M. Toscano, J. Phys. Chem. A, 2004, 108, 4916–4922 CrossRef CAS; (b) S. Jeremić, N. Filipović, A. Peulić and Z. Marković, Comput. Theor. Chem., 2014, 1047, 15–21 CrossRef PubMed.
- E. H. Anouar, S. Raweh, I. Bayach, M. Taha, M. S. Baharudin, F. D. Meo, M. H. Hasan, A. Adam, N. H. Ismail, J. F. Weber and P. Trouillas, J. Comput.-Aided Mol. Des., 2013, 27, 951–964 CrossRef CAS PubMed.
- (a) E. Klein, V. Lukeš and M. Ilčin, Chem. Phys., 2007, 336, 51–57 CrossRef CAS PubMed; (b) J. Rimarčik, V. Lukeš, E. Klein and M. Ilčin, J. Mol. Struct.: THEOCHEM, 2010, 952, 25–30 CrossRef PubMed.
- T. Tunç, M. Sarı, M. Sadıkoĝlu and O. Büyükgüngör, J. Chem. Crystallogr., 2009, 39, 672–676 CrossRef.
- C. Kontogiorgis and D. Hadjipavlou-Litina, J. Enzyme Inhib. Med. Chem., 2003, 18, 63–69 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. J. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, A. D. Malick, K. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle and J. A. Pople, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- Y. Zhao, N. E. Schultz and D. G. Truhlar, J. Chem. Theory Comput., 2006, 2, 364–382 CrossRef.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- (a) V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS; (b) V. Barone, M. Cossi, N. Rega and G. Scalmani, J. Comput. Chem., 2003, 24, 669–681 CrossRef PubMed.
- (a) J. E. Carpenter and F. Weinhold, J. Mol. Struct.: THEOCHEM, 1988, 169, 41–62 CrossRef; (b) A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- Z. Marković, D. Milenković, J. Đorović and S. Jeremić, J. Serb. Soc. Comp. Mech., 2013, 7, 1–9 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of compounds 1–10: bond lengths and angles, NBO charges and spin density, 1H NMR spectra, melting points. See DOI: 10.1039/c5ra02134k |
| This journal is © The Royal Society of Chemistry 2015 |