Green protection of pyrazole, thermal isomerization and deprotection of tetrahydropyranylpyrazoles, and high-yield, one-pot synthesis of 3(5)-alkylpyrazoles†
Basil M. Ahmed and
Gellert Mezei*
Department of Chemistry, Western Michigan University, Kalamazoo, Michigan, USA. E-mail: gellert.mezei@wmich.edu
First published on 26th February 2015
Abstract
We report a new synthetic approach that opens up the possibility of large scale, one-pot pyrazole derivatization by a wide variety of functionalities, including alkyl, halogen, hydroxyl, amino, azido, carbonyl, and organo-element (e.g., B, Si, P) groups. The approach is illustrated by the highly efficient synthesis of fourteen 3(5)-alkylpyrazoles, including the novel isopentyl- and n-hexadecyl derivatives, as well as 1,6-bis(pyrazol-3(5)-yl)hexane. The value of the new approach lies in the discovery of a green (solvent- and catalyst-free, quantitative) protection of pyrazole, followed by a high-yield lithiation/alkylation/deprotection sequence in the same pot. For the first time, the corresponding N-tetrahydropyran-2-yl (THP) intermediates have been isolated and characterized. Thermal isomerization of the 5-alkyl-1-(THP) to the 3-alkyl-1-(THP) isomer is shown to be an advantageous, green alternative to the acid-catalyzed, sequential protecting-group switching methodology in pyrazole chemistry. The X-ray crystal structures of 1,6-bis(pyrazol-3(5)-yl)hexane and 5-n-hexadecyl-1-(tetrahydropyran-2-yl)pyrazole reveal supramolecular architectures that shine light on the remarkable affinity for water and unexpected insolubility in organic solvents of alkylene-bridged bis(pyrazoles). 3(5)-Alkylpyrazoles are obtained in high yield from pyrazole by a one-pot procedure.
1. Introduction
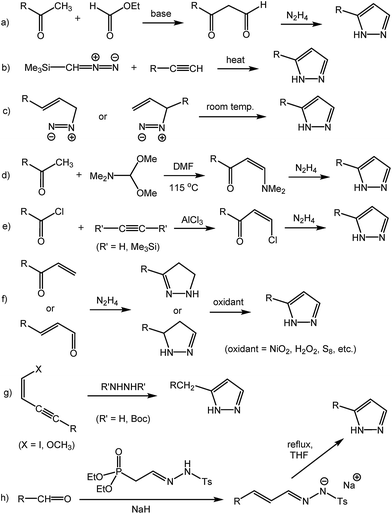
Pyrazole, a five-membered aromatic heterocycle with two adjacent nitrogen atoms (1,2-diazole), is a remarkably versatile moiety with a wide range of applications. Pyrazole-based molecules can be found in living organisms: currently, at least sixteen natural products containing the pyrazole moiety are known.1 3(5)-Nonylpyrazole, the first pyrazole-containing natural product derivative obtained in 1954, has the strongest antibiotic activity among 3(5)-alkylpyrazoles.2 4-Methylpyrazole is an effective antidote for methanol and ethylene glycol poisoning.3 A remarkably broad range of medicinal products (including anti-inflammatory, antitumor, antihypertensive, anticoagulant, antimicrobial, antiviral, anticonvulsant, antidepressant drugs), as well as agricultural chemicals (insecticides, fungicides, herbicides) and dyes (including food colorings), are pyrazole derivatives.4 Pyrazole-based ligands are also intensively used in various areas of coordination and materials chemistry.5In light of the widespread interest in pyrazole derivatives, we herein present a novel, straightforward and efficient synthesis of 3(5)-alkylpyrazoles, which offers valuable advantages over the currently used methods. 3(5)-Alkylpyrazoles are most commonly synthesized by the two archetypal pyrazole synthesis methods of Knorr6 and Pechmann.7 In a typical Knorr pyrazole synthesis, a β-ketoaldehyde (or the corresponding acetal/ketal), prepared from ethyl (or methyl) formate and a ketone, is condensed with hydrazine (Scheme 1a).2,8 The Pechmann pyrazole synthesis consists of the 1,3-dipolar cycloaddition of diazomethane to alkynes (Scheme 1b).9,10 3-Diazoalkenes spontaneously undergo intramolecular 1,3-dipolar cycloaddition to yield 3(5)-alkylpyrazoles (Scheme 1c).11 Other common routes to 3(5)-alkylpyrazoles free of 4-alkyl or 3,5-dialkyl side products include the conversion of a ketone to a β-(dimethylamino)vinyl ketone using N,N-dimethylformamide dimethyl acetal (Scheme 1d),10 or of an acyl chloride to a β-chlorovinyl ketone using acetylene (or its bis-trimethylsilyl derivative, Scheme 1e),12 followed in both cases by reaction with hydrazine. Enones or enals result in 2-pyrazolines upon reaction with hydrazine,10,13 which can be converted to the corresponding pyrazoles by oxidation (Scheme 1f).10,14 Alternatively, the corresponding ynones or ynals (Scheme 1f, alkyne instead of alkene) yield 3(5)-alkylpyrazoles directly upon treatment with hydrazine.15 Iodo-16 or methoxyenynes17 (Scheme 1g) also form 3(5)-alkylpyrazoles with hydrazine. Aldehydes can be transformed into 3(5)-alkylpyrazoles via α,β-unsaturated tosylhydrazones (Scheme 1h).18 All these methods present a number of shortcomings, including the need for not readily available and/or expensive starting materials or reagents (which often require additional synthesis and purification steps), highly toxic and/or potentially explosive reagents (diazomethane and derivatives, hydrazine), and low overall yields.
2. Results and discussion
2.1. Green pyrazole protection
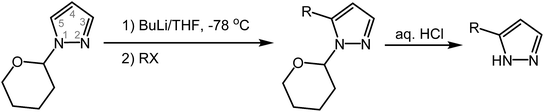
Herein we describe a pyrazole 3(5)-C-alkylation method that employs inexpensive 1H-pyrazole and 1-haloalkanes as starting materials, via a lithiated pyrazole intermediate (Scheme 2). Since C-alkylation requires protection of the pyrazole NH nitrogen (to avoid N-alkylation), a facile and efficient protection and deprotection method, using an inexpensive protecting group that will not undergo lithiation itself with nBuLi, is needed to maximize the overall yield and the value of the method. The tetrahydropyran-2-yl (THP) moiety proves to be an excellent protecting group to this end. The THP protecting group is usually introduced under acidic catalysis (e.g., F3CCOOH, p-toluenesulfonic acid), followed by work-up/purification involving either vacuum distillation from NaH19 or column chromatography.20 We find that the reaction of neat pyrazole with a slight excess of inexpensive 3,4-dihydro-2H-pyran (DHP) proceeds without a catalyst at 125 °C to provide 1-(tetrahydropyran-2-yl)pyrazole with 100% conversion (Scheme 3). The excess DHP can easily be removed at the end of the reaction under reduced pressure (b.p. 86 °C at 760 mmHg). Pure THP-protected pyrazole (by 1H and 13C NMR) is thus obtained quantitatively under solvent-free conditions, without the need for work-up or purification. The inspiration for this catalyst-free, solventless method originated from the observation that when heated, 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles isomerize to 3-alkyl-1-(tetrahydropyran-2-yl)pyrazoles, a process in which the THP–N bond is thermally cleaved, followed by the formation of a new THP–N bond at the adjacent, sterically less hindered N-atom (vide infra). As temperature increases, so does the acidity of the pyrazole N–H proton, eliminating the need for an additional acid catalyst.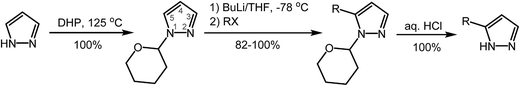 | ||
| Scheme 2 Synthesis of 3(5)-alkylpyrazoles by alkylation of pyrazole (DHP = 3,4-dihydro-2H-pyran; R = primary alkyl group; X = I or Br). | ||
 | ||
| Scheme 3 Proposed mechanism of formation of 1-(THP)pyrazole by electrophilic addition of pyrazole to 3,4-dihydro-2H-pyran (DHP). | ||
2.2. Lithiation/alkylation
Lithiation of THP-protected pyrazole with nBuLi at −78 °C, followed by the addition of a primary haloalkane, leads to 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles with excellent conversions, as shown by 1H NMR (Table 1). As expected for SN2 nucleophilic substitutions in general, this alkylation works best with least hindered, primary alkyl halides. Indeed, close to quantitative conversions (94–99%) are obtained with 1-iodo- and even with 1-bromoalkanes (except for C16). Under identical experimental conditions, secondary alkyl and vinyl iodides provide low conversions (cyclohexyl: 16%; isopropyl: 7%; sec-butyl: <1%; vinyl: 7%), and no reaction is observed with tert-butyl iodide and iodobenzene. Refluxing the reaction mixture under nitrogen does not provide a significant improvement. Allyl- and benzyl bromides give conversions of 7% and 36%, respectively. Isobutyl iodide (1-iodo-2-methylpropane), although a primary alkyl iodide, also provides a poor conversion (7%), further evidencing that steric crowding around the electrophilic reaction center is a critical limiting factor. Isopentyl iodide (1-iodo-3-methylbutane), in which the branching is one carbon atom further from the reaction center, reacts efficiently, with conversion similar to the n-butyl and n-pentyl analogs.| Entry | Halide reactant (RX) (Method) | Protected pyrazole | Pyrazole | Pyrazole | ||
|---|---|---|---|---|---|---|
| Conversion (%) | Isolated yield (%) | Conversion (%) | Isolated yield (%) | |||
| a Mixture of 5-alkyl and 3-alkyl isomers. | ||||||
| 1 | Iodomethane (A) | 100 | 99 |  |
100 | 94 |
| 2 | Iodoethane (A) | 99 | 89 |  |
100 | 97 |
| 3 | Bromoethane (A) | 96 | 90 | |||
| 4 | 1-Iodopropane (A) | 95 | 90 |  |
100 | 99 |
| 5 | 1-Iodobutane (A) | 96 | 95 |  |
100 | 92 |
| 6 | 1-Iodopentane (A) | 94 | 88 |  |
100 | 89 |
| 7 | 1-Iodo-3-methylbutane (A) | 94 | 91 |  |
100 | 99 |
| 8 | 1-Iodohexane (A) | 96 | 96 |  |
100 | 95 |
| 9 | 1-Bromohexane (A) | 96 | 92 | |||
| 10 | 1-Iodoheptane (A) | 96 | 91a | 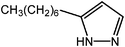 |
100 | 98 |
| 11 | 1-Iodoheptane (B) | 100 | 98 | |||
| 12 | 1-Iodooctane (A) | 96 | 89a |  |
100 | 99 |
| 13 | 1-Iodooctane (B) | 100 | 92 | |||
| 14 | 1-Iodononane (A) | 95 | 92a |  |
100 | 99 |
| 15 | 1-Iodononane (B) | 100 | 88 | |||
| 16 | 1-Bromodecane (A) | 96 | 91 |  |
100 | 94 |
| 17 | 1-Iodododecane (A) | 95 | Not isolated |  |
100 | 99 |
| 18 | 1-Iodododecane (B) | 100 | 92 | |||
| 19 | 1-Iodohexadecane (A) | 84 | 76 |  |
100 | 99 |
| 20 | 1-Iodohexadecane (B) | 82 | Not isolated | |||
| 21 | 1,6-Diiodohexane (B) | 100 | 80 |  |
100 | 84 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on a silica gel column.
1) on a silica gel column.A search of the literature reveals only two isolated reports when 3(5)-alkylpyrazoles were prepared directly from pyrazole: 3(5)-methylpyrazole (55% yield)21 and 3(5)-n-butylpyrazole (41% yield).22 The relatively low yields are partly due to the competing lithiation/alkylation of the protecting groups used in those two cases, 1-pyrrolidinomethyl21 and 4-methoxybenzyl,22 respectively. The tetrahydropyran-2-yl protecting group is inert under our working conditions, and reactivity at the pyrazole 5-position (adjacent to the protecting group, Scheme 2) is observed exclusively. Similarly, only 3-methyl-1-(tetrahydropyran-2-yl)pyrazole and not 5-methyl-1-(tetrahydropyran-2-yl)pyrazole can be deprotonated with nBuLi under identical conditions.23 Although the presence of the oxygen heteroatom (donor) in THP might facilitate lithiation24 through the “complex induced proximity effect” (CIPE),25 it has been noted earlier (without explanation) that lithiation by nBuLi of 1-methyl- or 1-phenylpyrazole, where CIPE is absent, likewise does not occur at the 3-position.26 The reason for the observed reactivity is presumably the repulsion between the lone pair of electrons of the carbanion formed upon deprotonation and the one of the adjacent nitrogen atom (Scheme 4). A similar phenomenon was later observed in a related imidazole system, and was termed “adjacent lone pair effect”.27 More recent experimental measurements, as well as computational studies confirm the significantly higher acidity of the proton in the 5-position than the one in the 3-position in 1-methylpyrazole28 and 1-arylpyrazoles (pKa difference of ∼7 for the latter).29
2.3. Thermal isomerization and deprotection of 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles
As mentioned above, the small amounts of unreacted 1-(tetrahydropyran-2-yl)pyrazole can be removed from the reaction mixture by heating to 55–60 °C in vacuum (0.10–0.20 mmHg). When alkyl = methyl, ethyl, n-propyl, n-butyl and i-pentyl, the excess iodoalkane can be removed as well, at temperatures well below the boiling point of the corresponding THP-protected pyrazoles. The latter can be distilled unaffected within a temperature range of 71–100 °C at 0.10–0.20 mmHg (see Experimental section). In the case of the n-pentyl, n-hexyl and n-heptyl derivatives, the corresponding excess iodoalkanes can be completely removed by heating to 55–60 °C under vacuum; however, distillation of the products at 104–106 °C (0.10 mmHg), 121–123 °C (0.20 mmHg) and 128–130 °C (0.15 mmHg), respectively, leads to isomerization (Scheme 5), and provides a mixture of the 5-alkyl and 3-alkyl isomers in a 64/36, 56/44 and 38/62 molar ratio, respectively, as determined by 1H NMR. In the case of n-octyl and longer n-alkyl derivatives, the excess 1-iodoalkane can no longer be removed from the reaction mixture by vacuum distillation (at 0.10–0.20 mmHg) without affecting the product: heating to a temperature at which the corresponding 1-iodoalkane vaporizes does not only lead to isomerization, but also to partial or even complete loss of the protecting group. For example, in the case of the n-octyl derivative, isomerization and partial deprotection occur at 80 °C, well below its boiling point (110–130 °C at 0.20 mmHg), at which extensive deprotection is observed. Loss of the tetrahydropyranyl group from THP-protected hydroxyl- or carboxylic acid-functionalized polymers at high temperatures has been reported.30Thermal isomerization occurs not only for 5-alkyl derivatives with longer alkyl chains, but also for the ones with short alkyl groups (and presumably even for the parent 1-(tetrahydropyran-2-yl)pyrazole), although at more elevated temperatures. For example, in the case of 5-methyl-1-(tetrahydropyran-2-yl)pyrazole, thermal isomerization at 125 °C in a pressure tube yields 32 mol% 3-methyl isomer after 24 hours, and 77 mol% 3-methyl isomer after 48 hours. No further change occurs after heating for 84 hours, indicating that equilibrium between the 5- and 3-methyl isomers is reached at a 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 molar ratio.
77 molar ratio.
As discussed above, only 3-alkyl-1-(THP)pyrazoles and not 5-alkyl-1-(THP)pyrazoles can be lithiated by nBuLi, if further derivatization of the pyrazole nucleus is desired. Therefore, the conversion of 5-alkyl- to 3-alkyl isomers is an important step for synthetic purposes. Pyrazole protecting-group switching is currently performed by acid-catalyzed, sequential deprotection–reprotection.20 Direct protecting group switching, under acidic catalysis, has also been reported.31 Thermal isomerization, presented herein, offers a new green alternative, which eliminates the need for solvents, additional reagents and work-up.
2.4. Acid-catalyzed deprotection of 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles
Complete deprotection of 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles can easily be achieved at ambient conditions, using an aqueous HCl solution, leading to pure 3(5)-alkylpyrazoles in near quantitative yields after standard work-up (Table 1).2.5. One-pot synthesis
The virtually quantitative conversion of the steps described above allows for three steps (protection, lithiation/alkylation, deprotection) to be performed sequentially in one pot, with no need for separation and purification of the intermediates. Thus, 3(5)-alkylpyrazoles can be prepared from pyrazole in excellent yields (see Experimental section).2.6. Crystallographic studies
The unexpected insolubility of 1,6-bis(pyrazol-3(5)-yl)hexane in most common solvents, as well as the difficulty in completely removing water from it, even after heating in high vacuum, prompted us to carry out single-crystal X-ray diffraction studies to elucidate its solid state structure.Within the crystal lattice of 1,6-bis(pyrazol-3(5)-yl)hexane, the bridging hexylene unit is fully extended and the two pyrazole moieties are at a dihedral angle of 63.9(1)° (Fig. 1). The pyrazole N–H hydrogen atoms are located on the N-atom adjacent to the bridging hexylene unit, and are involved in hydrogen-bonding with the solvent water molecule (Table 2). The two H-atoms of H2O, in turn, are involved in hydrogen-bonding to the other pyrazole N-atom. Besides the hydrogen-bonded network within the resulting two-dimensional layer (Fig. 2), edge-to-face interactions are observed between neighboring pyrazole (pz) units, with closest contact of 2.961(3) Å (C11–H11⋯N2′), C11–H11⋯centroid (pz) distance of 3.183(3) Å, centroid (pz)–centroid (pz) distance of 5.262(2) Å, and pz–pz dihedral angle of 63.9(1)°. Further edge-to-face pyrazole interactions are observed in-between the layers (Fig. 3), with closest contact of 2.901(4) Å (C2–H2⋯C12′), C2–H2⋯centroid (pz) distance of 3.169(3) Å, centroid (pz)–centroid(pz) distance of 5.159(2) Å, and pz–pz dihedral angle: 63.9(1)°. Such a 2D layered structure is unusual for NH–pyrazoles with no additional donor atoms, which typically self-assemble either into discrete cyclic motifs (dimers, trimers, tetramers, hexamers), or 1D catemers, via intermolecular N–H⋯N hydrogen bonds.32
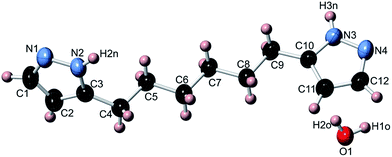 | ||
| Fig. 1 Crystal structure (thermal ellipsoid plot, 50% probability level) of 1,6-bis(pyrazol-3(5)-yl)hexane hydrate. | ||
| D–H⋯A | D–H (Å) | H⋯A (Å) | D⋯A (Å) | D–H–A (°) | Symmetry operator for A |
|---|---|---|---|---|---|
| O1–H1o⋯N4′ | 0.86(2) | 1.95(2) | 2.807(3) | 175(2) | −x + 1, −y + 2, −z + 1 |
| O1–H2o⋯N1′ | 0.84(2) | 1.98(2) | 2.818(3) | 177(3) | −x, −y, −z + 1 |
| N2–H2n⋯O1′ | 0.86 | 1.97 | 2.826(2) | 171 | x, y − 1, z |
| N3–H3n⋯O1′ | 0.86 | 1.98 | 2.838(3) | 175 | x + 1, y, z |
 | ||
| Fig. 2 Hydrogen-bonding network (green dashed lines) within a layer of 1,6-bis(pyrazol-3(5)-yl)hexane hydrate. | ||
 | ||
| Fig. 3 View of the layered structure of 1,6-bis(pyrazol-3(5)-yl)hexane hydrate (H-bonds shown by green dashed lines). | ||
1,6-Bis(pyrazol-3(5)-yl)hexane is only soluble in highly polar solvents, such as methanol, ethanol, dimethylformamide, dimethylsulfoxide, nitromethane and nitrobenzene, and is slightly soluble in cold water (solubility increases significantly in hot water). Its very poor solubility in other organic solvents (isopropanol, acetonitrile, ethyl acetate, acetone, tetrahydrofuran, diethyl ether, dichloromethane, chloroform, hydrocarbons), even at reflux temperatures, is surprising, given that 3(5)-propylpyrazole (half of the 1,6-bis(pyrazol-3(5)-yl)hexane molecule) is readily soluble in all common solvents, including hexane. The water of crystallization provides increased lattice enthalpy, by forming an extended hydrogen-bonded 2D network with the 1,6-bis(pyrazol-3(5)-yl)hexane units, which concurs with the difficulty in drying 1,6-bis(pyrazol-3(5)-yl)hexane. Furthermore, the extensive H-bonding interactions within the lattice are in accord with the poor solubility of the compound in solvents of medium or low polarity. THP-protected 1,6-bis(pyrazol-3(5)-yl)hexane, which lacks the N–H hydrogen bond donor, is readily soluble in common organic solvents (even in hexane, slightly), and similarly to all other 3(5)-alkylpyrazoles studied here, it does not have affinity for water.
Two crystallographically independent molecules are found within the asymmetric unit of 5-n-hexadecyl-1-(tetrahydropyran-2-yl)pyrazole (Fig. 4). The C16 chains are fully extended, a common feature of crystal structures of molecules possessing long hydrocarbon chains. An analysis of the Cambridge Structural Database reveals that it is the ratio between the size/number of the aliphatic chains and the size of the polar part of the molecule/structure that determines the conformation of the chain. When the size of the chain(s) dominates, a fully extended conformation of the chain, which maximizes the London dispersion forces between neighboring chains, provides a significant contribution to the overall crystal lattice enthalpy. The result is a layered structure, wherein the non-polar chains and the more polar part of the molecule each aggregate separately to form alternating 2D layers (Fig. 5). When the size of the polar part of the molecule (which might include counterions) dominates, the long hydrophobic chains might not be fully extended and densely packed, but can assume different bent conformations to fill the crystal lattice voids determined by the dominant polar interactions.33
 | ||
| Fig. 5 Packing diagram of 5-n-hexadecyl-1-(tetrahydropyran-2-yl)pyrazole (view down the b axis), showing a layered structure. | ||
3. Summary
The known synthetic strategies for the synthesis of 3(5)-alkylpyrazoles involve highly toxic and potentially explosive reagents, expensive starting materials, or long synthetic schemes with low overall yields, which render the synthesis of 3(5)-alkylpyrazoles expensive, often inefficient, and not readily scalable. Herein we present a novel one-pot strategy based on inexpensive and readily available starting materials (1H-pyrazole, haloalkanes) and reagents (3,4-dihydro-2H-pyran, nBuLi, aqueous HCl) that is easily scalable and eliminates additional intermediate synthesis and purification steps, provides high yields and minimizes waste production. We developed a new solventless, catalyst-free method for the protection of pyrazole, which needs no work-up or purification and is amenable for large-scale production, offering a truly green protection technique for 1H-pyrazole and its derivatives. Since 1-bromoalkanes were found to give similar conversions to 1-iodoalkanes (for C1–C12), we conclude that the use of the former is preferable, for they are less expensive, have lower boiling points allowing an easier removal of excess amounts from reaction mixtures (method A), and eliminate the need for washing with thiosulfate solution (to remove traces of iodine formed by oxidation of iodide by air, when 1-iodoalkanes are used as starting materials). In the case of longer alkyl derivatives, the use of excess 1-(tetrahydropyran-2-yl)pyrazole is preferable (method B), which is easier to remove under reduced pressure than the long-chain haloalkanes.We have shown that protecting-group switching (5-alkyl-1-(THP)pyrazole to 3-alkyl-1-(THP)pyrazole conversion) can be accomplished by simple, catalyst- and solvent-free thermal isomerization. The isomerization equilibrium favors the sterically less hindered 3-alkyl-1-THP isomer, and the ratio of 3-alkyl- vs. 5-alkyl-1-THP isomer increases with increasing alkyl chain length. Also, larger alkyl groups render the N–THP bond more labile, and as a result, isomerization (and even deprotection) occurs at lower temperatures.
We illustrated the utility of the new one-pot method by synthesizing and characterizing a series of 3(5)-alkylpyrazoles (C1–C10, C12), including the novel isopentyl- and n-hexadecyl derivatives, as well as 1,6-bis(pyrazol-3(5)-yl)hexane, and their corresponding N-tetrahydropyran-2-yl derivatives. The method has a wide scope, as other electrophiles, such as halogens, oxygen, tosyl azide, various carbonyl-containing compounds and organoelement (B, Si, P, etc.) reagents can be employed instead of alkyl halides, offering a wide variety of pyrazole derivatives for the pharmaceutical industry, agricultural applications and coordination/materials chemistry.
4. Experimental section
4.1. General
THF was dried with Na/benzophenone and freshly distilled under nitrogen prior to use. All other commercial reagents and solvents were used as received. Vacuum was measured with a McLeod gauge connected to a Schlenk line. Melting points were determined on a MEL-TEMP II (Laboratory Devices, USA) apparatus. 1H and 13C NMR spectra were recorded at room temperature on a Jeol JNM-ECP400 instrument. High-resolution mass spectra were obtained with a Waters Synapt G1 HDMS instrument, using an electrospray ionization source (negative mode for pyrazoles, positive mode for THP-protected pyrazoles).4.2. Synthesis of 1-(tetrahydropyran-2-yl)pyrazole (1)
A heavy-wall glass pressure flask was charged with pyrazole (50.0 g, 73.4 mmol) and 3,4-dihydro-2H-pyran (68.2 g, 81.1 mmol). The mixture was homogenized and then heated to 125 °C for 24 hours in an oven. After cooling to room temperature, the product was subjected to vacuum to remove traces of excess DHP. Pure THP-protected pyrazole (as indicated by 1H and 13C NMR) is obtained in quantitative yield (111 g). The product can be distilled at 64–65 °C (0.08 mmHg), if further purification is desired. 1H NMR (400 MHz, CDCl3): 7.60 (d, 1H, 3J = 2.2 Hz, 5-H-pz), 7.55 (s, 1H, 3-H-pz), 6.29 (m, 1H, 4-H-pz), 5.38 (dd, 1H, 3J = 9.9 Hz, 3J = 2.6 Hz, CH–THP), 4.02–4.07 (m, 1H, CH2O–THP), 3.65–3.73 (m, 1H, CH2O–THP), 1.97–2.19 (m, 3H, CH2–THP), 1.55–1.76 (m, 3H, CH2–THP) ppm. 1H NMR (400 MHz, DMSO-d6): 7.86 (d, 1H, 3J = 2.2 Hz, 5-H-pz), 7.48 (s, 1H, 3-H-pz), 6.30 (s, 1H, 4-H-pz), 5.39 (dd, 1H, 3J = 9.9 Hz, 3J = 2.6 Hz, CH–THP), 3.88–3.95 (m, 1H, CH2O–THP), 3.57–3.67 (m, 1H, CH2O–THP), 2.03–2.14 (m, 1H, CH2–THP), 1.85–1.97 (m, 2H, CH2–THP), 1.58–1.72 (m, 1H, CH2–THP), 1.48–1.58 (m, 2H, CH2–THP) ppm. 13C{1H} NMR (101 MHz, CDCl3): 139.7, 127.6, 106.1, 87.6, 67.9, 30.6, 25.0, 22.6 ppm.4.3. General procedure for the preparation of 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles (2–15)
Method A: 1-(tetrahydropyran-2-yl)pyrazole (5.000 g, 32.85 mmol) is dissolved in anhydrous THF (50 mL) in a Schlenk flask under a dry N2 atmosphere. The solution is chilled to −78 °C by stirring for 15 minutes in a dry-ice/isopropanol bath. nBuLi (1.6 M in hexane, 21 mL, 32.85 mmol) is added dropwise from an N2-purged syringe. Stirring at −78 °C is continued for another 30 minutes, then the 1-iodo- or 1-bromoalkane (36.14 mmol; in the case of 1,6-diiodohexane: 10.95 mmol) is added dropwise over 20 minutes. After stirring at −78 °C for 3 hours, the solution is left to warm up to room temperature and is quenched with water (1 mL). The volatiles are removed on a Rotavap, 80 mL water is added to the residue and it is extracted with ethyl acetate (or diethyl ether) (3 × 80 mL), followed by washing with a 10% aqueous sodium thiosulfate solution (80 mL; only when 1-iodoalkane is used as starting material), brine (80 mL) and drying with anhydrous MgSO4. After removing the volatiles on the Rotavap, the residual oil is heated to ∼75 °C (only when alkyl is C1–C6) under high vacuum (∼0.15 mmHg) to remove traces of the unreacted 1-(tetrahydropyran-2-yl)pyrazole and excess 1-haloalkane. Pure 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles (by 1H and 13C NMR) are thus obtained. When alkyl is C1–C4, the products can be distilled in vacuum, if needed. When alkyl is C5 and longer, distillation leads to isomerization and/or deprotection. In those cases, purification is accomplished by column chromatography (see example for C16 below). 1,6-Bis(1-(tetrahydropyran-2-yl)pyrazol-5-yl)hexane is purified by recrystallization from ethyl acetate/hexane. The products are colorless oils (C1–C9) or colorless crystalline solids (C10, C12, C16, 1,6-bis(1-(tetrahydropyran-2-yl)pyrazol-5-yl)hexane). For conversion calculations, distinct proton signals in the 1H NMR spectrum are used: 3-H-pyrazole, 4-H-pyrazole or CH–THP signals for Method A, and R–CH2–X (R = alkyl, X = I or Br) signals for Method B.Method B: same as Method A, except that 1-(tetrahydropyran-2-yl)pyrazole is used in 10% excess instead of the iodoalkane.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 3.8 g 1-iodohexadecane was recovered as the first fraction, followed by 1.1 g of a mixture of 1-iodohexadecane and 1-(tetrahydropyran-2-yl)pyrazole, and finally by 9.4 g pure 5-n-hexadecyl-1-(tetrahydropyran-2-yl)pyrazole. Rf = 0.25 (TLC). 1H NMR (400 MHz, CDCl3): 7.45 (d, 1H, 3J = 0.8 Hz, 3-H-pz), 6.04 (d, 1H, 3J = 0.8 Hz, 4-H-pz), 5.24 (dd, 1H, 3J = 10.1 Hz, 3J = 2.4 Hz, CH–THP), 4.00–4.06 (m, 1H, CH2O–THP), 3.59–3.67 (m, 1H, CH2O–THP), 2.58–2.72 (m, 2H, CH2(CH2)14CH3), 2.44–2.55 (m, 1H, CH2–THP), 2.07–2.14 (m, 1H, CH2–THP), 1.90–1.97 (m, 1H, CH2–THP), 1.54–1.78 (m, 5H, CH2–THP and CH2CH2(CH2)13CH3), 1.18–1.42 (m, 26H, (CH2)2(CH2)13CH3), 0.87 (t, 3H, 3J = 6.8 Hz, (CH2)15CH3) ppm. 13C NMR (101 MHz, CDCl3): 144.1, 139.1, 104.9, 84.2, 67.9, 32.0, 29.8 (seven overlapping peaks), 29.64, 29.59, 29.47 (two overlapping peaks), 29.43, 28.7, 25.3, 25.1, 23.0, 22.8, 14.2 ppm. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H44N2NaO 399.3351; found 399.3350.
1). 3.8 g 1-iodohexadecane was recovered as the first fraction, followed by 1.1 g of a mixture of 1-iodohexadecane and 1-(tetrahydropyran-2-yl)pyrazole, and finally by 9.4 g pure 5-n-hexadecyl-1-(tetrahydropyran-2-yl)pyrazole. Rf = 0.25 (TLC). 1H NMR (400 MHz, CDCl3): 7.45 (d, 1H, 3J = 0.8 Hz, 3-H-pz), 6.04 (d, 1H, 3J = 0.8 Hz, 4-H-pz), 5.24 (dd, 1H, 3J = 10.1 Hz, 3J = 2.4 Hz, CH–THP), 4.00–4.06 (m, 1H, CH2O–THP), 3.59–3.67 (m, 1H, CH2O–THP), 2.58–2.72 (m, 2H, CH2(CH2)14CH3), 2.44–2.55 (m, 1H, CH2–THP), 2.07–2.14 (m, 1H, CH2–THP), 1.90–1.97 (m, 1H, CH2–THP), 1.54–1.78 (m, 5H, CH2–THP and CH2CH2(CH2)13CH3), 1.18–1.42 (m, 26H, (CH2)2(CH2)13CH3), 0.87 (t, 3H, 3J = 6.8 Hz, (CH2)15CH3) ppm. 13C NMR (101 MHz, CDCl3): 144.1, 139.1, 104.9, 84.2, 67.9, 32.0, 29.8 (seven overlapping peaks), 29.64, 29.59, 29.47 (two overlapping peaks), 29.43, 28.7, 25.3, 25.1, 23.0, 22.8, 14.2 ppm. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H44N2NaO 399.3351; found 399.3350.4.4. General procedure for the deprotection of 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles (16–29)
5-Alkyl-1-(tetrahydropyran-2-yl)pyrazole (20 mmol) is dissolved in ethanol (200 mL) and conc. HCl (10 mL) is added dropwise under stirring. After stirring at room temperature for 8 hours, complete deprotection is indicated by the absence of 5-alkyl-1-(tetrahydropyran-2-yl)pyrazole signals in the 1H NMR spectrum of the reaction mixture. The volatiles are removed on a Rotavap at 35 °C and the residual aqueous solution is neutralized with NaHCO3 to pH ∼8, followed by extraction with diethyl ether (3 × 100 mL) and drying of the combined organic layers with anhydrous MgSO4. After removing the solvent and drying under high vacuum, pure 5-alkylpyrazoles (by 1H and 13C NMR) are obtained, that can be further purified by vacuum distillation or recrystallization (solid products). In the case of 1,6-bis(1-(tetrahydropyran-2-yl)pyrazol-5-yl)hexane, neutralization with NaHCO3 provides a white precipitate, which is filtered, washed with brine, dried, dissolved in hot nitrobenzene, filtered, and, after removal of the solvent, dried at 100 °C under high vacuum. The products are colorless oils, with the exception of the C16 derivative and 1,6-bis(pyrazol-5-yl)hexane, which are colorless crystalline solids.4.5. One-pot synthesis
(a) Pyrazole (10.000 g, 0.1469 mol) and 3,4-dihydro-2H-pyran (16.75 mL, 15.44 g, 0.1836 mol) are added to a 500 mL round-bottom pressure flask, which is sealed with a PTFE front seal bushing. The pyrazole dissolves completely in the 3,4-dihydro-2H-pyran and provides a homogeneous solution. The flask is placed in an oven and kept at 125 °C for 24 hours. After cooling to room temperature, the excess unreacted 3,4-dihydro-2H-pyran is removed under vacuum. (b) The flask containing pure 1-(tetrahydropyran-2-yl)pyrazole is evacuated and purged with N2. Anhydrous THF (300 mL) is added via N2 purged syringe, and the solution is chilled to −78 °C under stirring for 30 minutes. nBuLi (1.6 M in hexanes, 92 mL, 0.15 mol) is added drop-wise over 90 minutes. After stirring for an additional 30 minutes at −78 °C, 1-haloalkane (Method A: 0.1616 mol; Method B: 0.1335 mol) is added via syringe. The solution is stirred at −78 °C for 3 hours, then is left to warm up to room temperature overnight. The THF solvent and the excess haloalkane (Method A) or excess 1-THP-pyrazole (Method B) are removed under vacuum. (c) Deprotection is accomplished by dissolving the 5-alkyl-1-(tetrahydropyran-2-yl)pyrazoles in EtOH (300 mL) and stirring with 37% HCl (50 mL) for 4 hours. The solvent is removed under vacuum at 35 °C, the residue is neutralized with saturated aqueous NaHCO3 solution (to pH ∼8) and is extracted with diethyl ether (5 × 100 mL). The combined organic fractions are washed with brine (100 mL) and dried over anhydrous MgSO4. If 1-iodoalkane was used in the second step, additional washing with a dilute aqueous sodium thiosulfate solution is employed before drying with MgSO4, to remove iodine contamination. After filtration and evaporation of the solvent, the product is dried in vacuum (for longer alkyl chains the drying is carried out at 75 °C, to remove any remaining traces of haloalkane). Pure 3(5)-alkylpyrazoles (by 1H-NMR) are thus obtained, which can be further purified by vacuum distillation. For 3(5)-hexylpyrazole (obtained by Method A from 1-bromohexane), yield: 20.536 g (92%). For 3(5)-decylpyrazole (obtained by Method B from 1-bromodecane), yield: 27.675 g (91%).4.6. X-ray crystallography
Crystals of 1,6-bis(pyrazol-3(5)-yl)hexane hydrate were grown by slow cooling of a hot aqueous solution to which a small amount of methanol was added. Crystals of 5-n-hexadecyl-1-(tetrahydropyran-2-yl)pyrazole were grown by slow evaporation of a methanolic solution containing a small amount of water. X-ray diffraction data were collected at room temperature from a single-crystal mounted atop a glass fiber with cyanoacrylate glue, with a Bruker SMART APEX II diffractometer using graphite-monochromated Mo-Kα (λ = 0.71073 Å) radiation. The structures were solved by employing SHELXT intrinsic phasing methods and refined by full-matrix least squares on F2, using the APEX2 v2014.9-0 software package.34 All non-H atoms were refined with independent anisotropic displacement parameters. Hydrogen atoms were placed at calculated positions and refined using a riding model, except for the water hydrogens, which were located from the Fourier difference density maps and refined using a riding model with O–H distance restraints.† Crystallographic details are summarized in Table 3.| Hpz(CH2)6pzH·H2O | CH3(CH2)15pzTHP | |
|---|---|---|
| Formula | C12H20N4O | C24H44N2O |
| FW (g mol−1) | 236.32 | 376.61 |
| Crystal system | Triclinic | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 8.8013(4) | 8.7407(5) |
| b (Å) | 9.2025(4) | 9.4611(5) |
| c (Å) | 10.1004(5) | 29.7000(15) |
| α (deg) | 107.833(3) | 92.436(3) |
| β (deg) | 93.710(4) | 91.591(3) |
| γ (deg) | 114.352(3) | 104.178(3) |
| V (Å3) | 692.06(6) | 2377.3(2) |
| Crystal size | 0.07 × 0.15 × 0.40 | 0.10 × 0.35 × 0.70 |
| Z | 2 | 4 |
| Dcalc (g cm−3) | 1.134 | 1.052 |
| μ (mm−1) | 0.076 | 0.063 |
| θ Range (deg) | 2.60–24.12 | 2.01–34.60 |
| Reflns. collected | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 883 883 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 311 311 |
| Obsd. reflns. [I > 2σ(I)] | 1433 | 5456 |
| Data/restraints/parameters | 2194/2/160 | 8085/0/489 |
| GOF (on F2) | 0.956 | 1.015 |
| R factor [I > 2σ(I)] | R1 = 0.0473, wR2 = 0.1275 | R1 = 0.0422, wR2 = 0.1055 |
| R factor (all data) | R1 = 0.0835, wR2 = 0.1577 | R1 = 0.0696, wR2 = 0.1253 |
| Maximum peak/hole (e Å−3) | 0.128/−0.185 | 0.134/−0.126 |
Acknowledgements
We thank the U.S. National Science Foundation for financial support under Grant No. CHE-1404730.References
- V. Kumar, K. Kaur, G. K. Gupta and A. K. Sharma, Eur. J. Med. Chem., 2013, 69, 735–753 CrossRef CAS PubMed.
- T. Kosuge and H. Okeda, J. Biochem., 1954, 41, 183–186 CAS.
- (a) J. Brent, N. Engl. J. Med., 2009, 360, 2216–2223 CrossRef CAS PubMed; (b) L. I. Velez, G. Shepherd, Y. C. Lee and D. C. Keyes, J. Med. Toxicol., 2007, 3, 125–128 CrossRef.
- (a) S. Roy, S. Roy and G. W. Gribble, Top. Heterocycl. Chem., 2012, 29, 155–260 CAS; (b) F. K. Keter and J. Darkwa, Biometals, 2012, 25, 9–21 CrossRef CAS PubMed; (c) S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111, 6984–7034 CrossRef CAS PubMed; (d) A. Schmidt and A. Dreger, Curr. Org. Chem., 2011, 15, 1423–1463 CrossRef CAS.
- (a) E. M. Zueva, R. Herchel, S. A. Borshch, E. V. Govor, W. M. C. Sameera, R. McDonald, J. Singleton, J. Krzystek, Z. Trávníček, Y. Sanakis, J. E. McGrady and R. G. Raptis, Dalton Trans., 2014, 43, 11269–11276 RSC; (b) I. R. Fernando, S. A. Surmann, A. A. Urech, A. M. Poulsen and G. Mezei, Chem. Commun., 2012, 48, 6860–6862 RSC; (c) J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS PubMed; (d) M. Viciano-Chumillas, S. Tanase, L. J. De Jongh and J. Reedijk, Eur. J. Inorg. Chem., 2010, 3403–3418 CrossRef CAS; (e) M. A. Halcrow, Dalton Trans., 2009, 2059–2073 RSC; (f) R. Mukherjee, Coord. Chem. Rev., 2000, 203, 151–218 CrossRef CAS; (g) D. L. Reger, Comments Inorg. Chem., 1999, 21, 1–28 CrossRef CAS; (h) G. L. Monica and G. A. Ardizzoia, Progr. Inorg. Chem., 1997, 46, 151–238 CrossRef; (i) S. Trofimenko, Progr. Inorg. Chem., 1986, 34, 115–210 CrossRef CAS.
- L. Knorr, Chem. Ber., 1883, 16, 2597–2599 CrossRef.
- H. von Pechmann, Chem. Ber., 1898, 31, 2950–2951 CrossRef CAS.
- (a) G. D. Prestwich and M. S. Collins, J. Chem. Ecol., 1982, 8, 147–161 CrossRef CAS PubMed; (b) J. A. Jiménez, R. M. Claramunt, C. Escolástico and J. Elguero, Struct. Chem., 2000, 11, 77–83 CrossRef; (c) N. R. Easton, U. S. Patent US 2992163, 1961; (d) G. Karmas and R. A. Mallory, 1959; (e) W. Franke and R. Kraft, Chem. Ber., 1953, 86, 797–800 CrossRef CAS.
- I. Zrinski, M. Juribasic and M. Eckert-Maksic, Heterocycles, 2006, 68, 1961–1967 CrossRef CAS.
- (a) T. Wang, J. F. Kadow, N. A. Meanwell, K.-S. Yeung, Z. Zhang, Z. Yin, Z. Qiu, D. H. Deon, C. A. James, E. H. Ruediger and C. Bachand, U.S. Patents US 0063744 A1 and US 0186292 A1, 2004; (b) T. Wang, Z. Zhang, N. A. Meanwell, J. F. Kadow, Z. Yin, Q. M. Xue, A. Regueiro-Ren, J. D. Matiskella and Y. Ueda, U.S. Patent US 0110785 A1, 2004.
- J. L. Brewbaker and H. Hart, J. Am. Chem. Soc., 1969, 91, 711–715 CrossRef CAS.
- D. Dehe, C. Lothschütz and W. R. Thiel, New J. Chem., 2010, 34, 526–532 RSC.
- (a) N.-W. Huber, H.-J. Niclas, U. Radics and W. Kristof, Ger. Patent DE 19826669 B4, 2007; (b) H. R. Merkle and E. Fretschner, Ger. Patent DE 4328228 A1, 1995; (c) N. Yoshihara, T. Hasegawa and S. Hasegawa, Bull. Chem. Soc. Jpn., 1991, 64, 719–720 CrossRef CAS.
- R. Fischer, T. Dockner, W. Rohr and E. Stroefer, Ger. Patent DE 3415385 A1, 1985.
- (a) N. V. Galapagos, Worldwide Patent WO 2007/138072 A2, 2007; (b) J. J. Hale, C. L. Lynch, C. G. Caldwell, C. A. Willoughby, D. Kim, D.-M. Shen, S. G. Mills, K. T. Chapman, L. Chen, A. Gentry, M. MacCoss and Z. D. Konteatis, U.S. Patent US 0094989 A1, 2002; (c) R. Grandi, U. M. Pagnoni and R. Trave, J. Chem. Res., Synop., 1979, 327 (J. Chem. Res., Miniprint, 1979, 3782–3789) CAS.
- R. Martín, M. Rodríguez Rivero and S. L. Buchwald, Angew. Chem., Int. Ed., 2006, 45, 7079–7082 CrossRef PubMed.
- M. Winter, Helv. Chim. Acta, 1963, 46, 1754–1760 CrossRef CAS.
- N. Almirante, A. Cerri, G. Fedrizzi, G. Marazzi and M. Santagostino, Tetrahedron Lett., 1998, 39, 3287–3290 CrossRef CAS.
- M. B. Young, J. C. Barrow, K. L. Glass, G. F. Lundell, C. L. Newton, J. M. Pellicore, K. E. Rittle, H. G. Selnick, K. J. Stauffer, J. P. Vacca, P. D. Williams, D. Bohn, F. C. Clayton, J. J. Cook, J. A. Krueger, L. C. Kuo, S. D. Lewis, B. J. Lucas, D. R. McMasters, C. Miller-Stein, B. L. Pietrak, A. A. Wallace, R. B. White, B. Wong, Y. Yan and P. J. Nantermet, J. Med. Chem., 2004, 47, 2995–3008 CrossRef CAS PubMed.
- A. D. Velankar, G. Quintini, A. Prabhu, A. Weber, G. Hunaeus, B. Voland, M. Wuest, C. Orjeda, D. Harel, S. Varghese, V. Gore, M. Patil, D. Gayke, M. Herdemann, I. Heit and A. Zaliani, Bioorg. Med. Chem., 2010, 18, 4547–4559 CrossRef CAS PubMed.
- A. R. Katritzky, G. W. Rewcastle and W.-Q. Fan, J. Org. Chem., 1988, 53, 5685–5689 CrossRef CAS.
- C. Subramanyam, Synth. Commun., 1995, 25, 761–774 CrossRef CAS.
- Y. Mo, B. M. Ahmed, L. Guan, J. Karty and G. Mezei, Org. Lett., 2014, 16, 4680–4683 CrossRef CAS PubMed.
- H. W. Gschwend and H. R. Rodriguez, Org. React., 1979, 26, 1–360 CAS.
- P. Beak and A. I. Meyers, Acc. Chem. Res., 1986, 19, 356–363 CrossRef CAS.
- P. W. Alley and D. A. Shirley, J. Am. Chem. Soc., 1958, 80, 6271–6274 CrossRef CAS.
- (a) Y. Takeuchi, H. J. C. Yeh, K. L. Kirk and L. A. Cohen, J. Org. Chem., 1978, 43, 3565–3570 CrossRef CAS; (b) Y. Takeuchi, K. L. Kirk and L. A. Cohen, J. Org. Chem., 1978, 43, 3570–3578 CrossRef CAS.
- (a) T. Balle, M. Begtrup, J. W. Jaroszewski, T. Liljefors and P.-O. Norrby, Org. Biomol. Chem., 2006, 4, 1261–1267 RSC; (b) S. M. Villano, A. J. Gianola, N. Eyet, T. Ichino, S. Kato, V. M. Bierbaum and W. C. Lineberger, J. Phys. Chem. A, 2007, 111, 8579–8587 CrossRef CAS PubMed.
- F. Chevallier, Y. S. Halauko, C. Pecceu, I. F. Nassar, T. U. Dam, T. Roisnel, V. E. Matulis, O. A. Ivashkevich and F. Mongin, Org. Biomol. Chem., 2011, 9, 4671–4684 CAS.
- (a) D. P. Sweat, M. Kim, A. K. Schmitt, D. V. Perroni, C. G. Fry, M. K. Mahanthappa and P. Gopalan, Macromolecules, 2014, 47, 6302–6310 CrossRef CAS; (b) A. Bolognesi, F. Galeotti, J. Moreau, U. Giovanella, W. Porzio, G. Scaviaab and F. Bertini, J. Mater. Chem., 2010, 20, 1483–1488 RSC; (c) X. Han, X. Chen, G. Vamvounis and S. Holdcroft, Macromolecules, 2005, 38, 1114–1122 CrossRef CAS; (d) S. Yang, J. Wang, K. Ogino, S. Valiyaveettil and C. K. Ober, Chem. Mater., 2000, 12, 33–40 CrossRef CAS; (e) J. Yu and S. Holdcroft, Macromolecules, 2000, 33, 5073–5079 CrossRef CAS; (f) S. Song, S. Chang, S. Takahara and T. Yamaoka, Polym. Adv. Technol., 1998, 9, 579–587 CrossRef CAS.
- M. McLaughlin, K. Marcantonio, C.-Y. Chen and I. W. Davies, J. Org. Chem., 2008, 73, 4309–4312 CrossRef CAS PubMed.
- (a) V. Bertolasi, P. Gilli, V. Ferretti, G. Gillia and C. Fernández-Castaño, Acta Crystallogr., Sect. A: Found. Crystallogr., 1999, B55, 985–993 CAS; (b) C. Foces-Foces, I. Alkorta and J. Elguero, Acta Crystallogr., Sect. A: Found. Crystallogr., 2000, B56, 1018–1028 CrossRef CAS PubMed; (c) L. Infantes and S. Motherwell, Struct. Chem., 2004, 15, 173–184 CrossRef CAS; (d) I. Alkorta, J. Elguero, C. Foces-Foces and L. Infantes, ARKIVOC, 2006, ii, 15–30 Search PubMed.
- S. Hayami, R. Moriyama, Y. Shigeyoshi, R. Kawajiri, T. Mitani, M. Akita, K. Inoue and Y. Maeda, Inorg. Chem., 2005, 44, 7295–7297 CrossRef CAS PubMed.
- APEX2 v2014.9-0, Bruker AXS Inc., Madison, WI, 2014 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR spectra of all intermediates and final products, CIF files for 1,6-bis(pyrazol-3(5)-yl)hexane hydrate and 5-n-hexadecyl-1-(tetrahydropyran-2-yl)pyrazole. CCDC 1040198 and 1040199. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra00837a |
| This journal is © The Royal Society of Chemistry 2015 |