Novel pH sensitive N-doped carbon dots with both long fluorescence lifetime and high quantum yield†
Abstract
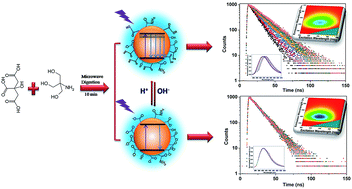
Novel N-doped carbon dots (CDs) were synthesized. The fluorescent quantum yield of the CDs is up to 93.3%, and the fluorescence lifetime of the CDs is up to 19.50 ns. The optical properties of the CDs in various pH solutions were probed in different ways. The fluorescence characteristics of the CDs depended on change of surface structure, which was associated with pH value.

 Please wait while we load your content...
Please wait while we load your content...