One-pot synthesis of NiAl–CO3 LDH anti-corrosion coatings from CO2-saturated precursors†
Yi Liu‡
ab,
Tongwen Yu‡ac,
Rui Cai*a,
Yanshuo Lia,
Weishen Yang*a and
Jürgen Carob
aState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, China. E-mail: cairui@dicp.ac.cn; yangws@dicp.ac.cn
bInstitute of Physical Chemistry and Electrochemistry, Leibniz University Hannover, Callinstr. 3A, D-30167 Hannover, Germany
cUniversity of Chinese Academy of Sciences, Beijing, 100039, China
First published on 19th March 2015
Abstract
Anti-corrosive coatings based on layered double hydroxides (LDHs) have been considered as promising alternatives to conventional chromate-containing conversion coatings. Among various LDHs, carbonate-intercalated LDH coatings with a c-axis preferred orientation should be the optimum structure for protecting metals against corrosion. Herein we successfully prepared NiAl–CO3 LDH coatings on aluminium plates in one step. Particularly it was found that CO2 dissolved in the precursor solution exerted great influence on the microstructure and anti-corrosion capacity of prepared LDH coatings. Trace amounts of CO2 in the precursor solution led to the formation of ab-oriented 7 μm-thick LDH coatings, while preferentially c-oriented LDH coatings with an average thickness of 12 μm were formed from CO2-saturated precursor solutions. A DC polarization test demonstrated that preferentially c-oriented LDH coatings exhibited much higher anti-corrosion performance than ab-oriented LDH coatings possibly due to the decreased density of mesoscopic defects. Simultaneously, CO2, the green gas, was also positively utilized.
Introduction
Corrosion is the gradual destruction of materials (usually metals) by chemical reaction with their environment. Corrosion of metal and its alloys often leads to a series of economic and safety issues. Chromate-containing conversion coatings, which have historically been evaluated as an effective anticorrosion method for metallic substrates,1 are currently restricted due to their toxic and carcinogenic properties.2 As an alternative, various new materials have been developed.3 Among them, layered double hydroxide (LDH) coatings on metal surfaces provide a cost-effective and eco-friendly way to protect metals (like Mg, Al and their respective alloys) against corrosion.4 LDHs are a series of layered compounds consisting of positively charged brucite-like sheets, coupled with charge-compensating anions located in interlayer regions.5 Compositional flexibility in both positively charged layers and charge-balancing anions leads to functional diversity.6Using aluminum as a probe substrate, F. Z. Zhang recently reported the synthesis of a laurate-intercalated ZnAl LDH film which involved an anion exchange step.4a The prepared LDH film showed a corrosion current density (abbreviated as Icorr) as low as 10−9 A cm−2 in a direct current (DC) polarization test. X. Duan prepared a ZnAl–NO3 LDH film7 and further revealed that the film not only acted as an anti-corrosive coating but also initiated a self-healing process via the dissolution–recrystallization process upon exposure to a chloride-containing environment.8
The most striking feature of LDHs is their compositional flexibility. In terms of anti-corrosive capacity, carbonate-intercalated LDHs should be the optimum choice. This is because the ion-exchange equilibrium constants of charge-compensating anions follow the sequence CO32− > SO42− > OH− > F− > Cl− > Br− > NO3−.9 In principle, once a carbonate-intercalated LDH film is formed, most of the corrosive components, like the Cl−, SO42−, Br− and F− anions commonly found in water, will not easily exchange these carbonate anions, thereby retarding the corrosion of the underlying metals.10 Besides chemical composition, also the crystallographic orientation of the LDH films may exert a strong influence on their anticorrosive capacity. Under ideal conditions, c-oriented LDH films are preferred as corrosion-resistant coatings, because in this case, plate-like LDH crystallites are arranged parallel to the substrate and thus will minimize intercrystalline voids and grain boundaries. In particular these boundaries may become inter-crystalline defects upon excessive exposure to the corrosive medium. Inspired by the above-mentioned principles, we report here a one-step, hydrothermal crystallization of preferentially c-oriented NiAl–CO3 LDH coatings on Al substrates. Prepared LDH coatings showed excellent anticorrosive performance, considerable long-term corrosion durability as well as strong mechanical stability (schematically shown in Fig. 1).
Experimental
Synthesis of NiAl–CO3 LDH films on Al substrates
Pure Al plates were supplied by Aldrich (99.99 wt%) at a size of 1.5 × 1.5 cm2 and a thickness of 0.2 mm.The precursor solution was prepared by adding 5.8 g Ni(NO3)2·6H2O (98.0 wt%, Merke) and 4.8 g NH4NO3 (98.0 wt%, Aldrich) into 100 ml CO2-saturated water (Vitalitasia Classic, containing saturated CO2). Consequently 10 ml NH3·H2O (1 wt%, Aldrich) was slowly added into the aqueous solution and stirred in an ice bath for 10 min.
An Al plate was first horizontally placed into a 50 ml Teflon-lined stainless vessel. 35 ml of the precursor solution was then poured into the vessel and sealed. The vessel was put into convective oven pre-heated to 85 °C. After an elapsed time of 40 h, the vessel was taken out and cooled to room temperature in air. Finally the film was washed with copious amounts of Distilled De-Ionized (DDI) water. Before the DC polarization test, the LDH film was dried in a convective oven at 60 °C for 12 h.
Besides CO2-saturated water, deionized (DI) water was also used as a solvent to prepare LDH films following the same procedure and recipe as shown above. Note: DI water was left in open air for at least 1 month to reach equilibrium of dissolved CO2 with the surrounding atmosphere.
DC polarization test
We employed a three-electrode system to carry out DC polarization test at room temperature on a Solartron SI 1287 electrochemical interface: NiAl–CO3 LDH film-coated Al samples or bare Al substrate as a working electrode, a gauze platinum counter electrode, and an Ag/AgCl electrode as the reference electrode.A 3.5 wt% aqueous solution of sodium chloride was used as the corrosive medium. Before the test, the samples were immersed in the corrosive medium to measure the open-circuit-potential for 30 min. The sweep rate, potential range of DC polarization test was −1.5–+2.5 V (vs. reference), 10 mV s−1.
Results and discussion
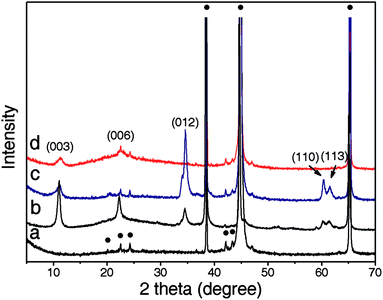
Recent studies revealed that NiAl brucite-like sheets had an extraordinarily high affinity for CO32− anions derived from dissolved CO2. For instance, our recent research revealed that the variation of the CO2 concentration in the precursor solution affected the microstructure and thus gas separation performance of supported NiAl–CO3 LDH membranes.11 CO32− anions located in the interlayer region were derived from CO2 dissolved in the precursor solution.12 In our approach, the precursor solution was prepared by mixing Ni(NO3)2·6H2O, NH4NO3, and NH3·H2O in a CO2-saturated H2O solvent. Then the aluminium substrate, which also served as the Al3+ source, was vertically placed into the solution before the chemical bath deposition. It was anticipated that the dissolved CO2 could act as a source of carbonate anions and participate in the formation of CO32− intercalated LDHs. For comparison, aged DI water was also used as the solvent. It was found that the concentration of dissolved CO2 in the precursor solution exerted significant influence on the microstructure of NiAl–CO3 LDH coatings. In the case of aged DI water [abbreviated as NiAl–CO3 LDH (DI aged)], which had a low concentration of dissolved CO2 (∼1.3 × 10−5 M),11 the Al substrate surface became rough and fully covered with plate-like LDH microcrystals ∼1 μm in size after the hydrothermal reaction (shown in Fig. 2a). Nevertheless, the formed LDH layer was not well-intergrown. Substantial intercrystalline defects were simultaneously generated and spread over the surface of the LDH film (Fig. 2b). The SEM cross-sectional view from Fig. 2c illustrated that the LDH layer was ∼7 μm thick. XRD pattern of the film showed five conspicuous diffraction peaks at 2θ values of 11.2°, 22.5°, 34.6°, 60.4° and 61.5° respectively (Fig. 3c), which was consistent with the reflections of (0 0 3), (0 0 6), (0 1 2), (1 1 0) and (1 1 3) crystal planes in NiAl–CO3 LDH powders (shown in ESI-1†).13 This confirmed that the formed layer indeed belonged to the LDH phase.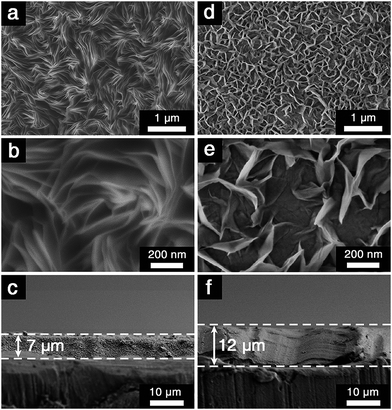 | ||
| Fig. 2 SEM images of NiAl–CO3 LDH films prepared from (a–c) aged DI water and (d–f) CO2-saturated water solvents, respectively. | ||
In contrast, the LDH layer synthesized from the CO2-saturated precursor solution [abbreviated as NiAl–CO3 LDH (CO2-satur.)], which contained a much higher concentration of dissolved CO2 (∼3.3 × 10−2 M),11 showed a distinct morphology. After in situ growth, the Al substrate had been fully covered with well-intergrown LDH crystallites with no visible defects (Fig. 2d), thus indicating that the new layer was highly compact. Higher magnification of the image demonstrated that the LDH crystal faces were well-developed and that the grain size was reduced to ∼200 nm (Fig. 2e). Nevertheless, the thickness of the LDH layer had reached ∼12 μm (Fig. 2f). Different from the NiAl–CO3 LDH (DI aged) film, only (0 0 3) and (0 0 6) diffraction peaks were found in the whole XRD pattern of the NiAl–CO3 LDH (CO2-satur.) film (Fig. 3d).
The different morphology of the NiAl–CO3 LDH (DI aged) and NiAl–CO3 LDH (CO2-satur.) layers is mainly attributed to the differences in the concentration of dissolved CO2 in the precursor solution. Owing to the much higher concentration of CO2 in the CO2-saturated precursor solution, the concentration of CO32−, which acts as the intercalating anions, is also higher, which will further increase both the nucleation density and growth rate of the NiAl–CO3 LDH film (as demonstrated in ESI-2†).11 In addition, the sufficient supply of CO2 also effectively suppresses the generation of intercrystalline defects in the NiAl–CO3 LDH layer during the in situ hydrothermal growth. In contrast, substantial defects are formed in the case of aged DI water (Fig. 2a) since the concentration of nutrient CO2 in the precursor solution is insufficient to guarantee complete sealing of the void spaces in the NiAl–CO3 LDH (DI aged) layer.
Preferred orientation of NiAl–CO3 LDH films was further investigated due to its potential impact on the anti-corrosive capacity. The intensity ratio I(0 0 3)/I(0 1 2) was employed here to evaluate the preferred orientation of LDH films,7 in which I(0 0 3) and I(0 1 2) represented peak heights of the (0 0 3) and (0 1 2) diffractions, respectively. A higher I(0 0 3)/I(0 1 2) value indicates that the LDH film has a c-axis preferred orientation, while a lower value demonstrates that prepared film is preferentially ab-oriented. Simultaneously, NiAl–CO3 LDH powders, which were scraped from corresponding LDH film samples, were also characterized as a reference (Fig. 3b). As was shown in the figure, all (0 0 3), (0 0 6), (0 1 2), (1 1 0) and (1 1 3) diffraction peaks were clearly observable and the I(0 0 3)/I(0 1 2) ratio reached 2.7 for the NiAl–CO3 LDH powders. As for the NiAl–CO3 LDH (DI aged) layer (Fig. 3c), this ratio was reduced significantly to 0.2, thus revealing that this film was dominantly ab-oriented and most LDH crystallites should be vertically aligned on the substrate (Fig. 2a). Additionally, a strong (1 1 0) diffraction peak at 2θ value of 60.4° further convinced its preferred ab-orientation. On the contrary, for the NiAl–CO3 LDH (CO2-satur.) layer, the (0 1 2) diffraction peak was totally undetectable (Fig. 3d) and the I(0 0 3)/I(0 1 2) ratio reached +∞. Although the relative low crystallinity of LDH crystallites in the NiAl–CO3 LDH (CO2-satur.) film may affect accurate evaluation of the preferred orientation of LDH films, it was believed that prepared LDH film was still preferentially c-oriented to a certain extent since after all, the (0 1 2) diffraction peak did not appear in the XRD pattern. The preferred c-orientation of the NiAl–CO3 (CO2-satur.) LDH layer could be further confirmed by its layer-like structure (Fig. 2f). Conventionally, c-oriented LDH films could be prepared by physical deposition of pre-formed LDH crystallites on substrates considering their plate-like shapes.14 Nevertheless, the adhesion between the film and substrate was very weak, making them unsuitable as anti-corrosive coatings.6b In comparison, in situ growth method provided better adhesion to the substrate. So far, most of LDH films prepared in this way showed random or ab-axis preferred orientation,15 the preparation of preferentially c-oriented LDH films, however, has turned out to be very difficult unless substrates were pre-modified with appropriate organic structure-directing agents.16 X. Duan et al. reported the in situ crystallization of ab-oriented and c-oriented MgAl–CO3 LDH films on bare and PVA-modified glass substrates, respectively.16 The ab-oriented LDH film was interpreted by the “evolution selection” growth mechanism. In such case the growth of LDH film was under kinetic control. On the contrary, the formation of preferentially c-oriented MgAl–CO3 LDH film on the PVA-modified glass side was attributed to the strong hydrogen bonding interactions between PVA and MgAl–CO3 LDH crystallites. The hydrogen-bonding energy, which referred to the thermodynamic parameter, indicated that the film formation process was in fact under thermodynamic control.
It was generally accepted that preferred orientation of inorganic crystalline films was driven by the interaction between thermodynamic and kinetic factors, as in the case of MFI zeolite films.17–20 Based on the previous research, in this study we try to elucidate the change in preferred orientation of NiAl–CO3 LDH coatings as follow: in most cases in situ hydrothermal growth of LDH films is a kinetically controlled process. Under this condition, prepared LDHs film will show ab-axis preferred orientation via the “evolution selection” mechanism as in the case of the NiAl–CO3 LDH (DI aged) coatings. In contrast, formation of c-oriented NiAl–CO3 LDH coatings is a thermodynamically favourable process since the most stable configuration for them to settle onto the surface is to lie on their flat surface (ab-face) parallel to substrates. Since concentration of CO2 in the CO2-saturated precursor solution is much higher (∼0.03 M), evolution of the NiAl–CO3 LDH (CO2-satur.) coatings is no longer under kinetic control and instead, thermodynamic factors gradually dominate the hydrothermal reaction, which ultimately leads to the formation of preferentially c-orientated NiAl–CO3 LDH films.
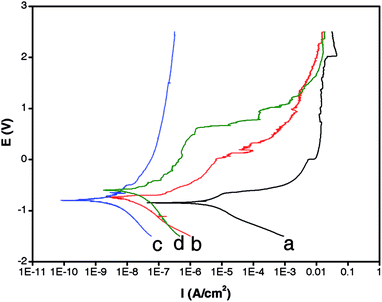
DC polarization is an effective tool for evaluating anti-corrosive coatings.21 In addition to the NiAl–CO3 LDH (DI aged) and LDH (CO2-satur.)-modified Al plates, a bare Al plate was also subject to the test for reference. Before the DC experiment, all samples were immersed in a corrosive medium (3.5 wt% aqueous NaCl solution) for 30 min. The horizontal-like anodic current curve of the Al substrate implied that it readily corroded in the NaCl solution.7 The Icorr for the bare Al substrate was ∼10−6 A cm−2 (Fig. 4a). After coating the Al plate with a NiAl–CO3 LDH (DI aged) layer, the Icorr was reduced by one order of magnitude (∼10−7 A cm−2, Fig. 4b). A further large decrease of Icorr to ∼10−9 A cm−2 could be achieved with the NiAl–CO3 LDH (CO2-satur.) coating (Fig. 4c), which was rarely observed previously for simple anion (like CO32−, NO3−, Cl− and CrO4−) intercalated LDH coatings22 and comparable with various organic anion (like laurate and 8-hydroxyquinoline) intercalated LDH coatings4a,22f (summarized in Table 1). This implied that NiAl–CO3 LDH (CO2-satur.) layers were competent as high-performance, anti-corrosive coatings. In addition, the much lower Icorr for the NiAl–CO3 LDH (CO2-satur.) film, when compared with the NiAl–CO3 LDH (DI aged) film, was expected because substantial defects were present in the latter (shown in ESI-2†).
| LDH film | Icorr bare | Icorr modified |
|---|---|---|
| (A cm−2) | (A cm−2) | |
| ZnAl–V10O28 LDH-epoxy22a | 10−6 | 10−7 |
| ZnAl–EDTA LDH-epoxy22b | 4.2 × 10−7 | 3.0 × 10−7 |
| ZnAl–CO3 LDH-epoxy22b | 4.2 × 10−7 | 3.9 × 10−7 |
| ZnAl–CrO4 LDH-epoxy22b | 4.2 × 10−7 | 0.7 × 10−7 |
| ZnAl–Cl LDH-epoxy22b | 4.2 × 10−7 | 2.5 × 10−7 |
| MgAl–CO3 LDH22c | 5.9 × 10−7 | 4.5 × 10−6 |
| MgAl–CO3 LDH22d | 6.3 × 10−6 | 1.5 × 10−6 |
| ZnAl–NO3 LDH4a | 10−6 | 10−8 |
| ZnAl–laurate LDH4a | 10−6 | 10−9 |
| MgAl–CO3 LDH22e | 1.9 × 10−4 | 4.9 × 10−6 |
| MgAl–NO3 LDH22f | 7.4 × 10−8 | 1.1 × 10−8 |
| MgAl–8HQ LDH22f | 7.4 × 10−8 | 0.9 × 10−9 |
| This work | 10−6 | 10−9 |
We further investigated the long-term durability of the NiAl–CO3 LDH (CO2-satur.) layer against corrosion. Results showed that even after immersion in a corrosive medium (3.5 wt% NaCl solution) for 15 days, the Icorr of the NiAl–CO3 LDH layer-coated Al plate remained as low as ∼10−8 A cm−2 (Fig. 4d). Simultaneously, the surface morphology of the LDH layer after the immersion process was characterized (shown in ESI-3†). Still no conspicuous cracking was observed in the NiAl–CO3 LDH (CO2-satur.) layer. In comparison, the bare Al plate had been badly corroded after immersion in the NaCl solution. It was supposed that both the intercalation of carbonate anions and preferred c-orientation of the NiAl–CO3 LDH (CO2-satur.) layer may contribute to the long-term durability.
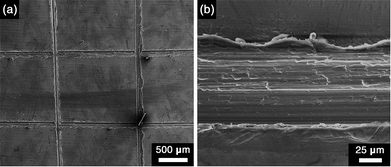
Strong adhesion of anti-corrosion coatings to the metal surface is of vital importance for their practical applications.21a,23 Herein scratch test was employed to evaluate the adhesion strength of NiAl–CO3 LDH (CO2-satur.) coatings to substrates. It was found that no peeling off or delamination occurred after the cross cutting through LDH coatings, which indicated strong adhesion of LDH coatings to aluminium plate surface (Fig. 5). The excellent anti-corrosive capacity, considerable long-term durability as well as strong adhesion of NiAl–CO3 LDH (CO2-satur.) coatings will make them find wide applications in the field of corrosion protection.
Conclusions
In summary, with the in situ hydrothermal method, we successfully prepared anti-corrosive NiAl–CO3 LDH coatings on aluminium substrates in one step. In particular, it was found that the use of CO2-saturated water led to the formation of defect-free and preferentially c-oriented NiAl–CO3 LDH layers, which showed excellent anticorrosive performance (polarization current density: ∼10−9 A cm−2), considerable long-term corrosion durability as well as strong adhesion to substrates. This LDH layer protection technique is simple and eco-friendly, as carbonate intercalated LDHs are conventionally synthesized by precipitation in NaOH–Na2CO3 suspension24 or hydrothermal decomposition of urea/HMT.25 Moreover, CO2, which is also called green gas, is positively utilized. It was further demonstrated that the anti-corrosive capacity of LDH films could be improved not only by appropriate selection of metal ions and charge compensating anions, but also through proper optimization of their microstructure as in the case of zeolite films.3a,b,21Acknowledgements
The authors are grateful to the CAS 100-talent program, GZ 911 Sino-German Cooperation Group on Inorganic Membranes, Natural Science Foundation of China (21176231, 21361130018 and 21476223) and Alexander von Humboldt Foundation for the financial support.Notes and references
- (a) J. Zhao, G. Frankel and R. L. McCreery, J. Electrochem. Soc., 1998, 145, 2258 CrossRef CAS PubMed; (b) L. Xia, E. Akiyama, G. Frankel and R. McCreery, J. Electrochem. Soc., 2000, 147, 2556 CrossRef CAS PubMed.
- (a) S. Pommiers, J. Frayret, A. Castetbon and M. Potin-Gautier, Corros. Sci., 2014, 84, 135 CrossRef CAS PubMed; (b) S. H. Zaferani, M. Peikari, D. Zaarei, I. Danaee, J. M. Fakhraei and M. Mohammadi, Corrosion, 2013, 69, 372 CrossRef CAS PubMed.
- (a) R. Cai, M. W. Sun, Z. W. Chen, R. Munoz, C. O'Neill, D. E. Beving and Y. S. Yan, Angew. Chem., Int. Ed., 2008, 47, 525 CrossRef CAS PubMed; (b) Y. J. Dong, Y. Peng, G. L. Wang, Z. B. Wang and Y. S. Yan, Ind. Eng. Chem. Res., 2012, 51, 3646 CrossRef CAS; (c) M. L. Zheludkevich, R. Serra, M. F. Montemor, K. A. Yasakau, I. M. M. Salvado and M. G. S. Ferreira, Electrochim. Acta, 2005, 51, 208 CrossRef CAS PubMed; (d) K. S. Wang, J. Unger, J. D. Torrey, B. D. Flinn and R. K. Bordia, J. Eur. Ceram. Soc., 2014, 34, 3597 CrossRef CAS PubMed.
- (a) F. Z. Zhang, L. L. Zhao, H. Y. Chen, S. L. Xu, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2008, 47, 2466 CrossRef CAS PubMed; (b) J. K. Lin, C. L. Hsia and J. Y. Uan, Scr. Mater., 2007, 56, 927 CrossRef CAS PubMed; (c) J. Wang, D. D. Li, X. Yu, X. Y. Jiang, M. L. Zhang and Z. H. Jiang, J. Alloys Compd., 2010, 494, 271 CrossRef CAS PubMed; (d) T. Stimpfling, F. Leroux and H. Hintze-Bruening, Appl. Clay Sci., 2013, 83–84, 32 CrossRef CAS PubMed.
- R. Z. Ma, J. B. Liang, X. H. Liu and T. Sasaki, J. Am. Chem. Soc., 2012, 134, 19915 CrossRef CAS PubMed.
- (a) Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124 CrossRef CAS PubMed; (b) X. X. Guo, F. Z. Zhang, D. G. Evans and X. Duan, Chem. Commun., 2010, 46, 5197 RSC; (c) B. Sels, D. D. Vos, M. Buntinx, F. Pierard, A. K.-D. Mesmaeker and P. Jacobs, Nature, 1999, 400, 855 CrossRef CAS PubMed; (d) G. R. Williams and D. O'Hare, J. Mater. Chem., 2006, 16, 3065 RSC; (e) S. P. Newman and W. Jones, New J. Chem., 1998, 22, 105 RSC; (f) V. Rives and M. A. Ulibarri, Coord. Chem. Rev., 1999, 181, 61 CrossRef CAS.
- X. X. Guo, S. L. Xu, L. L. Zhao, W. Lu, F. Z. Zhang, D. G. Evans and X. Duan, Langmuir, 2009, 25, 9894 CrossRef CAS PubMed.
- T. T. Yan, S. L. Xu, Q. Peng, L. L. Zhao, X. H. Zhao, X. D. Lei and F. Z. Zhang, J. Electrochem. Soc., 2013, 160, C480 CrossRef CAS PubMed.
- (a) S. Miyata, Clays Clay Miner., 1983, 31, 305 CAS; (b) Y. Israëli, G. Taviot-Guého, J.-P. Besse, J.-P. Morel and N. Morel-Fesrosiers, J. Chem. Soc., Dalton Trans., 2000, 791 RSC.
- J. Chen, Y. W. Song, D. Y. Shan and E. H. Han, Corros. Sci., 2012, 65, 268 CrossRef CAS PubMed.
- Y. Liu, N. Y. Wang and J. Caro, J. Mater. Chem. A, 2014, 2, 5716 CAS.
- H. Y. Chen, F. Z. Zhang, T. Chen, S. L. Xu, D. G. Evans and X. Duan, Chem. Eng. Sci., 2009, 64, 2617 CrossRef CAS PubMed.
- Z. P. Liu, R. Z. Ma, Y. Ebina, N. Iyi, K. Takada and T. Sasaki, Langmuir, 2007, 23, 861 CrossRef CAS PubMed.
- (a) J. H. Lee, S. W. Rhee and D. Y. Jung, Chem. Commun., 2003, 2740 RSC; (b) J. H. Lee, S. W. Rhee, H. J. Nam and D. Y. Jung, Adv. Mater., 2009, 21, 546 CrossRef CAS PubMed; (c) R. Z. Liang, R. Tian, W. Y. Shi, Z. H. Liu, D. P. Yan, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2013, 49, 969 RSC.
- (a) H. Y. Chen, F. Z. Zhang and X. Duan, Adv. Mater., 2006, 18, 3089 CrossRef CAS; (b) J. H. Lee, S. W. Rhee, H. J. Nam and D. Y. Jung, Adv. Mater., 2009, 21, 546 CrossRef CAS PubMed.
- X. X. Guo, F. Z. Zhang, S. L. Xu, D. G. Evans and X. Duan, Chem. Commun., 2009, 6836 RSC.
- S. Li, X. Wang, D. Beving, Z. W. Chen and Y. S. Yan, J. Am. Chem. Soc., 2004, 126, 4122 CrossRef CAS PubMed.
- (a) Z. P. Lai, G. Bonilla, I. Diaz, J. G. Nery, K. Sujaoti, M. A. Amat, E. Kokkoli, O. Terasaki, R. W. Thompson, M. Tsapatsis and D. G. Vlachos, Science, 2003, 300, 456 CAS; (b) A. Gouzinis and M. Tsapatsis, Chem. Mater., 1998, 10, 2497 CrossRef CAS.
- A.-J. Bons and P. D. Bons, Microporous Mesoporous Mater., 2003, 62, 9 CrossRef CAS.
- (a) Z. B. Wang and Y. S. Yan, Microporous Mesoporous Mater., 2001, 48, 229 CrossRef CAS; (b) Z. B. Wang and Y. S. Yan, Chem. Mater., 2001, 13, 1101 CrossRef CAS.
- (a) D. E. Beving, A. M. P. McDonnell, W. S. Yang and Y. S. Yan, J. Electrochem. Soc., 2006, 153, B325 CrossRef CAS PubMed; (b) E. P. Hu, Y. Li, W. Huang, Q. Yan, D. P. Liu and Z. P. Lai, Microporous Mesoporous Mater., 2009, 126, 81 CrossRef CAS PubMed.
- (a) R. G. Buchheit, H. Guan, S. Mahajanam and F. Wong, Prog. Org. Coat., 2002, 47, 174 CrossRef PubMed; (b) T. Stimpfling, F. Leroux and H. Hintze-Bruening, Appl. Clay Sci., 2013, 83–84, 32 CrossRef CAS PubMed; (c) J. Chen, Y. W. Song, D. Y. Shan and E. H. Han, Corros. Sci., 2011, 53, 3281 CrossRef CAS PubMed; (d) J. Wang, D. D. Li, X. Yu, X. Y. Jing, M. L. Zhang and Z. H. Jiang, J. Alloys Compd., 2010, 494, 271 CrossRef CAS PubMed; (e) F. Z. Zhang, M. Sun, S. L. Xu, L. L. Zhao and B. W. Zhang, Chem. Eng. J., 2008, 141, 362 CrossRef CAS PubMed; (f) L. D. Wang, K. Y. Zhang, H. R. He, W. Sun, Q. F. Zong and G. C. Liu, Surf. Coat. Technol., 2013, 235, 484 CrossRef CAS PubMed.
- Y. M. Liu, L. H. Li, X. Cai, Q. L. Chen, M. Xu, Y. W. Hu, T.-L. Cheung, C. H. Shek and P. K. Chu, Thin Solid Films, 2005, 493, 152 CrossRef CAS PubMed.
- Z. P. Xu, G. S. Stevenson, C. Q. Lu, G. Q. Lu, P. F. Bartlett and P. P. Gray, J. Am. Chem. Soc., 2006, 128, 36 CrossRef CAS PubMed.
- N. Iyi, K. Tamura and H. Yamada, J. Colloid Interface Sci., 2009, 340, 67 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details: XRD pattern of NiAl–CO3 LDH powders, magnified cross-sectional SEM images of NiAl–CO3 LDH films prepared from DI aged and CO2-saturated precursor solutions, SEM images of the LDH film after long-term corrosion in NaCl solution. See DOI: 10.1039/c5ra01969a |
| ‡ These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |