Comparative theoretical study of formic acid decomposition on PtAg(111) and Pt(111) surfaces
Yuanyuan Qi,
Jun Gao,
Dongju Zhang* and
Chengbu Liu
Key Lab of Colloid and Interface Chemistry, Ministry of Education, Institute of Theoretical Chemistry, Shandong University, Jinan, 250100, P. R. China. E-mail: zhangdj@sdu.edu.cn; Fax: +86-531-88564464; Tel: +86-531-88365833
First published on 9th February 2015
Abstract
Pt-based catalysts are known as the best electrocatalysts for formic acid (HCOOH) oxidation. Maximizing their use efficiency and reducing the CO poisoning effect are highly desirable, however, very challenging. Aiming at these interesting issues, this work presents a theoretical study of the catalytic decomposition of HCOOH on an ideal single-atom model catalyst of PtAg nanostructures, which consists of isolated Pt atoms anchored to an Ag(111) surface and is referred to as PtAg(111). The barrier of the rate-determining step of HCOOH decomposition to CO2 on PtAg(111) is calculated to be 0.38 eV, which is not significantly different from that on a pure Pt(111) surface, 0.35 eV. On the other hand, the barrier of HCOOH decomposing to CO on PtAg(111) is found to be higher than that on pure Pt(111), 0.83 vs. 0.67 eV. These results indicate that the single-atom PtAg(111) (Pt-decorated Ag(111) surface) presents promising catalytic performance for HCOOH oxidation, which promotes HCOOH dehydrogenation to CO2 as good as pure Pt(111) and inhibits HCOOH dehydration to undesirable CO that poisons the catalyst. The present results rationalize the experimental observation that Pt–Ag alloy electrocatalysts show improved catalytic performance toward HCOOH oxidation, and provide a clue for the rational design of Pt-based single-atom catalysts.
1. Introduction
The electrocatalytic oxidation of formic acid (HCOOH) has attracted lots of attention in recent decades due to its relevance to developing efficient direct formic acid fuel cells (DFAFCs).1–9 Pt and Pd noble metal nanoparticles supported commonly on various carbon materials such as carbon nanotubes10–14 are known as the most effective anode catalysts for the oxidation of formic acid at the anode of DFAFCs.15–20 Extensive investigations have been conducted to elucidate the mechanism of HCOOH electro-oxidation on the surfaces of various metals, including Pt,15,21–23 Pd,13,20,24,25 Ru,9,26,27 Au,28,29 and Ni,24,30 which have provided fundamental insights into the overall reaction pathways. However, the large scale commercial application of DFAFCs has been hindered by several technical and economical barriers. It is the most severe challenge on how to improve the poor performance of the durability of Pt and Pd catalysts, which is generally attributed to the poisoning effects of CO and CO-like species produced during HCOOH oxidation.21,31–34 Another big problem is how to maximizing the use efficiency of Pt and Pd catalysts, the most expensive materials. Modification of Pt and Pd catalysts with other metals, non-noble metals in particular, has turned out to be a feasible method to improve both the poisoning effect35–45 and the use efficiency. Two kinds of effects, the so-called geometrical ensemble effect35,46,47 and the electronic ligand effect,35,47–49 have been proposed to understand the improved catalytic performance of bimetal catalysts.In recent years, Pt-based bimetal catalysts, such as PtRu,35,50,51 PtPd,13,52 PtFe,53,54 and PtBi,22,38,44,55 have been studied extensively for HCOOH oxidation. Ag has been identified as a promising alloying element to enhance CO-poisoning tolerance of Pt catalysts, and PtAg bimetal nanoparticles have been demonstrated to show remarkable promotion of the CO-poisoning tolerance compared with pure Pt electrocatalysts.46,56,57
Although much is known about the preparations of different morphological PtAg bimetal catalysts, the mechanism of their improved CO-poisoning tolerance is still not understood well at the molecular level. Herein, this work presents a comparative theoretical study of formic acid decomposition on the bimetallic PtAg(111) and pure Pt(111) surfaces. The (111) surface is known as the most stable crystal plane of the fcc lattice, and thus is expected to contribute the most areas for the surface of bulk metals, alloys, and nanoparticles, and so is chosen for the present study. By performing density functional theory (DFT) calculations, we show the mechanism details of the elementary steps of HCOOH decomposition on two (111) surfaces, from which we hope to provide a reasonable explanation of the CO-poisoning tolerance enhancement of PtAg nanoparticles.
2. Model and computational details
Both the PtAg(111) surface and Pt(111) surface were modeled using a triple-layer p(3 × 3) slab comprising 3 metal layers with 9 atoms per layer and each slab was separated by a vacuum of 15 Å to guarantee the interaction between neighboring cells was negligible in the z direction. The PtAg(111) surface was built based on the Ag(111) surface, where three Ag atoms in the top layer were replaced by three Pt atoms, as shown in Fig. 1. In such a PtAg(111) surface model, the Pt atoms in the top layer were isolated into single Pt atoms and anchored to Ag(111) to follow the idea of maximizing the use efficiency of Pt materials. The water environment on the (111) surfaces was simulated with four explicit water molecules per unit cell. All DFT calculations were performed using the CASTEP code58,59 and the exchange–correlation effects were described with the generalized gradient approximation (GGA) using the PBE functional proposed by Perdew, Burke, and Ernzerhof.60 The electron wave functional was expanded by a plane-wave basis set with a cutoff energy of 400 eV. A 2 × 2 × 1 Monkhorst–Pack k-point grid was used for the integrations of the Brillouin zone. The transition states were searched using the linear and quadratic synchronous transit (LST/QST) procedure.58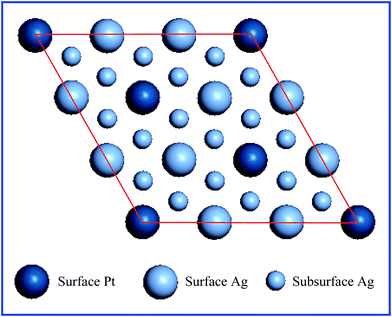 | ||
| Fig. 1 Top view of the p(3 × 3) unit cell of the PtAg(111) surface, the Pt and Ag atoms are shown in dark blue and light blue, respectively. The red lines are the edges of the unit cell. | ||
The performance of the three-layer slab model and the calculation parameters, including the p(3 × 3) supercell, 2 × 2 × 1 k-point, 15 Å vacuum zone, and the energy cutoff of 400 eV, have been tested extensively in previous studies.7,24,30,61,62 Using these parameters, the largest adsorption energy of HCOOH on the Pt(111) surface is calculated to be 0.43 eV. Increasing the slab thickness to 5 atomic layers with the top three layers relaxed, the calculated adsorption energy is improved to 0.39 eV, which is not remarkably different from the result using the three-layer slab model, indicating that the results obtained using the three-layer slab model are of acceptable accuracy. In addition, our main concern in the present work is the relative barriers involved in the different decomposition pathways of HCOOH, which can counteract most of the energy errors resulting from the limited slab thickness as well as the neglect of dipole corrections, spin polarization, and the dispersion effect.63,64 Thus we consider that the conclusion to be drawn based on relative energies is reliable.
3. Results and discussion
The catalytic decomposition of HCOOH on metal surfaces is generally thought to follow a dual-pathway mechanism65–69 consisting of indirect and direct pathways. The indirect pathway features adsorbed CO as the intermediate of HCOOH oxidation, and the direct pathway involves HCOOH reacting to CO2 without the participation of CO species. Clearly, the direct pathway is highly desirable while the indirect one is not wanted because it generates CO poisoning species and hence deactivates catalysts. So it is expected that the catalytic performance of a catalyst of HCOOH decomposition is directly related to the relative ease or difficulty of two pathways, which are generally evaluated using the energy barrier to be overcome to carry out a reaction pathway. In this sense, we calculated and compared the thermal dissociation barriers of HCOOH involved in the pathways forming CO and CO2 on PtAg(111) and Pt(111) surfaces. Previous studies7,58 indicated that an applied electrode potential to mimic the electro-catalytic oxidation of HCOOH has little effect on oxidation barriers. Therefore, the results presented in this work can serve as a foundational prototype to understand the complex electrochemical oxidation of HCOOH.3.1 HCOOH decomposition to CO2 on AgPt(111) and Pt(111)
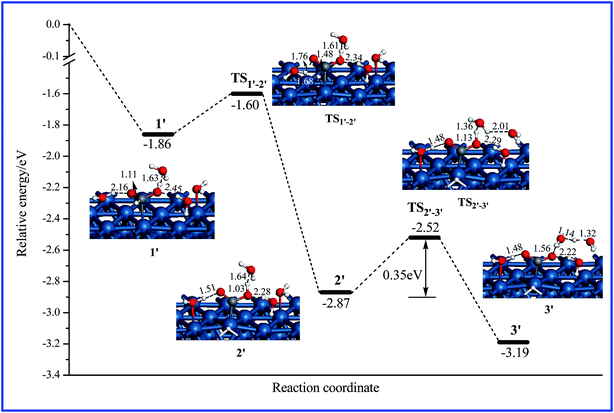
The present work located three energetically favorable pathways of HCOOH decomposition on PtAg(111) to form CO2. Fig. 2 shows the optimized geometries involved along each pathway, and Fig. 3 depicts calculated relative energy diagrams along the three pathways. Initially, HCOOH in its trans configuration prefers to lie parallel to the AgPt(111) surface to form more hydrogen bonds with the adjacent water molecules as illustrated in structure 1, which is the common precursor in pathways I–III. Along pathway I, to perform the subsequent transformation, the trans-HCOOH in 1 is first converted to its cis configuration in 2 via TS1–2 with a barrier of 0.39 eV. Then the first dehydrogenation step (the O–H bond scission) occurs via TS2–3, leading to HCOO + H in 3. The barrier involved in this process is calculated to be 0.33 eV. It is noted that the neighboring water molecule plays a substantial role for the O–H bond scission, which acts as a “bridge” assisting the H migration from HCOOH to the surface. In 3, the formate species HCOO is adsorbed on the surface with a C–H down configuration. The second dehydrogenation step (the C–H bond scission) occurs via TS3–4 with a barrier of 0.39 eV, leading to CO2 + H and completing the HCOOH decomposition. The overall reaction is calculated to be exothermic by 0.50 eV.In pathways II and III, the initially adsorbed HCOOH in 1 is first converted to a suitable configuration in 5 through TS1–5 with a barrier of 0.16 eV, where the C–H bond and/or O–H bond are geometrically ready for the upcoming scissions. Along pathway II, the first dehydrogenation step involves the C–H bond scission that proceeds via TS5–6 with a barrier of 0.56 eV, leading to the adsorbed COOH species in 6. The following second dehydrogenation step (the O–H bond scission) is found to be a barrierless process because the transition state (TS6–7) is located even lower in energy than its precursor (structure 6), indicating that once formed, COOH readily cleaves its O–H bond to form CO2 in 7. Alternatively, along pathway III, the HCOOH in 5 can cleave its O–H and C–H bonds simultaneously via TS5–8 with a barrier of 0.38 eV, resulting in the direct formation of CO2. From the relative energy profile shown in Fig. 3, one can see that pathway III is the dominant pathway for dehydrogenation of HCOOH to CO2.
From the geometries shown in Fig. 2, it is noted that (i) throughout the entire process of HCOOH decomposition, the reaction occurs at the Pt site rather than the Ag site, indicating Pt is the catalytic active center of the AgPt(111) surface; (ii) water molecules play a substantial role in the dehydrogenation process of HCOOH, and act as a bridge transferring H atoms from HCOOH to the surface.
Similarly, on Pt(111) the dehydrogenation processes of HCOOH to CO2 are also calculated to make a comparison with that on PtAg(111). For simplification, only the energetically most favorable pathway is given in Fig. 4. The C–H bond scission first occurs followed by the O–H bond scission, and the calculated barriers of these two dehydrogenation steps are 0.26 and 0.35 eV, respectively. These barriers are not remarkably different from the corresponding ones (0.16 and 0.38 eV) on PtAg(111) shown in Fig. 3, implying that the PtAg(111) can perform as well as Pt(111) for the catalytic dehydrogenation of HCOOH to CO2. The present PtAg(111) model can be described as a Pt-decorated Ag(111), in which Pt atoms were isolated into single Pt atoms and anchored to Ag(111) to follow the idea of maximizing the use efficiency of Pt materials. From an atom-economical point of view, such a catalyst is much more inexpensive than a pure Pt catalyst.
3.2 HCOOH decomposition to CO on AgPt(111) and Pt(111)
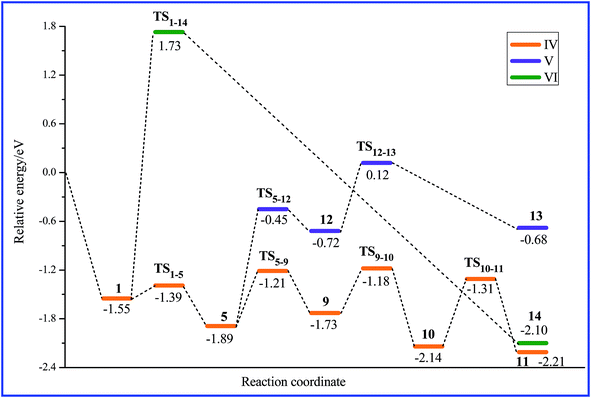
Fig. 5 shows three possible pathways (IV, V and VI) of HCOOH decomposition to CO on PtAg(111), and Fig. 6 gathers optimized geometries involved in these pathways. From the calculated relative energy profiles in Fig. 6, pathway IV is the energetically most favorable. As shown in Fig. 5, the transformation from 1 to 5 is also the initial step of pathways IV and V, like that discussed above for the formation of CO2. Along pathway IV, the next step is the C–H bond scission via TS5–9 with a barrier of 0.68 eV, leading to COOH and H. The C–H distance (1.61 Å) in TS5–9 clearly indicates that the C–H bond is breaking, leading to a bidentate absorbed COOH species in 9. The following transformation from 9 to 10 corresponds to the migration of the H atom adsorbed on the surface to a water molecule. In such a process, the hydroxyl O plays a role of “bridge” transferring H atoms. The barrier from 9 to 10 is calculated as 0.55 eV. The COOH species in 10 serves as the direct precursor for the C–OH bond scission through TS10–11, where the C–OH distance (2.08 Å) clearly identifies the rupture of the C–OH bond, leading to the formation of CO. The barrier of this process is calculated to be 0.83 eV, which is the highest barrier along pathway IV, implying that the C–OH bond scission is the rate-determining step for the decomposition of HCOOH to CO.Along pathway V, the adsorbed HCOOH in 5 first breaks its C–OH bond through TS5–12 to form CHO and OH species in 12, and then the C–H bond scission of the CHO species leads to the formation of CO. These two processes proceed through TS5–12 and TS12–13, respectively, where the C–OH distance (2.06 Å) and C–H distance (1.46 Å) clearly identify the characteristics of the breaking C–OH and C–H bonds. The calculated barrier is 1.44 eV for the C–OH bond scission and 0.84 eV for the C–H bond scission. These values are higher than those in pathway IV, where the C–H bond scission occurs prior to the C–OH bond scission. Pathway VI is an energetically much less favorable pathway for the formation of CO. It is a concerted process of the C–OH bond rupture and C–H bond rupture via TS1–14. The barrier of this process is calculated to be as high as 3.28 eV, implying an impossible process.
Again, to make a comparison with the results on pure Pt(111), we performed the corresponding calculations and the relevant results along the most favorable pathway are shown in Fig. 7. It is found that the initial C–H scission with a barrier of 0.67 eV is the rate-determining step for the decomposition of HCOOH to CO on Pt(111). Compared to pathway IV in Fig. 6, the most favorable pathway on PtAg(111) with the rate-determining step barrier of 0.83 eV, it is clear that the formation of CO on PtAg(111) becomes more difficult, which is in agreement with the observed reduced CO-poisoning effect of PtAg bimetallic catalysts for HCOOH oxidation.
Comparing Fig. 2 with 5, it is noted that the decomposition of HCOOH to CO2 generally needs only one single Pt site, while that to CO requires not only Pt but also the neighboring Ag, which is less active than Pt and hence gives PtAg(111) better performance on durability.
4. Conclusions
In summary, by performing periodic DFT calculations, we have presented a comparative theoretical study of HCOOH decomposition on the PtAg(111) and Pt(111) surfaces. The PtAg(111) surface also presents an ideal model of single-atom Pt-based catalysts. The calculated results indicate that on PtAg(111), HCOOH decomposition to CO2 involves a barrier of 0.38 eV, which is not significantly different from that on pure Pt(111), 0.35 eV, implying the PtAg(111) can perform as well as pure Pt(111) for HCOOH decomposition to CO2. On the other hand, the calculations confirm that HCOOH decomposition to CO on PtAg(111) becomes more difficult than that on the pure Pt(111) surface, as is shown by the calculated barriers, 0.83 and 0.67 eV, in the two situations, respectively. This is in agreement with the improved catalytic efficiency of PtAg bimetallic alloy nanoparticles towards HCOOH oxidation. The present theoretical results also provide a clue for the rational design of single-atom Pt-based catalysts.Acknowledgements
We greatly acknowledge the financial support from National Natural Science Foundation of China (nos 21433006, 21273131, 21373124 and 91127014), and Specialized Research Fund for the Doctoral Program of Higher Education (no. 20130131110012).References
- A. Capon and R. Parson, J. Electroanal. Chem. Interfacial Electrochem., 1973, 44, 1–7 CrossRef CAS.
- A. Capon and R. Parsons, J. Electroanal. Chem. Interfacial Electrochem., 1973, 44, 239–254 CrossRef CAS.
- A. Capon and R. Parsons, J. Electroanal. Chem. Interfacial Electrochem., 1973, 45, 205–231 CrossRef CAS.
- C. Rice, S. Ha, R. I. Masel, P. Waszczuk, A. Wieckowski and T. Barnard, J. Power Sources, 2002, 111, 83–89 CrossRef CAS.
- J. S. Kim, J. K. Yu, H. S. Lee, J. Y. Kim, Y. C. Kim, J. H. Han, I. H. Oh and Y. W. Rhee, Korean J. Chem. Eng., 2005, 22, 661–665 CrossRef CAS.
- Y. Zhu, Z. Khan and R. I. Masel, J. Power Sources, 2005, 139, 15–20 CrossRef CAS PubMed.
- M. Neurock, M. Janik and A. Wieckowski, Faraday Discuss., 2009, 140, 363 RSC.
- H. F. Wang and Z. P. Liu, J. Phys. Chem. C, 2009, 113, 17502–17508 CAS.
- W. Y. Yu, G. M. Mullen, D. W. Flaherty and C. B. Mullins, J. Am. Chem. Soc., 2014, 136, 11070–11078 CrossRef CAS PubMed.
- J. H. Li, C. T. Ke, K. H. Liu, P. Li, S. H. Liang, G. Finkelstein, F. Wang and J. Liu, ACS Nano, 2014, 8, 8564–8572 CrossRef CAS PubMed.
- J. H. Li, K. H. Liu, S. B. Liang, W. W. Zhou, M. Pierce, F. Wang, L. M. Peng and J. Liu, ACS Nano, 2013, 8, 554–562 CrossRef PubMed.
- J. Zeng, J. Yang, J. Y. Lee and W. Zhou, J. Phys. Chem. B, 2006, 110, 24606–24611 CrossRef CAS PubMed.
- B. Liu, H. Y. Li, L. Die, X. H. Zhang, Z. Fan and J. H. Chen, J. Power Sources, 2009, 186, 62–66 CrossRef CAS PubMed.
- L. M. Chen, D. Ma, Z. Zhang, Y. Y. Guo, D. Q. Ye and B. C. Huang, ChemCatChem, 2012, 4, 1960–1967 CrossRef CAS.
- R. Holze and A. M. Castro Luna, J. Appl. Electrochem., 1988, 18, 679–686 CrossRef CAS.
- F. Godinez Salomon, E. Arce Estrada and M. Hallen Lopez, Int. J. Electrochem. Sci., 2012, 7, 2566–2576 CAS.
- J. Guo, Y. Y. Gao, C. H. Tan, Y. P. Li, S. L. Zhao, L. Z. Bai and S. Y. Zhang, Fuel Cells, 2013, 13, 167–172 CrossRef CAS.
- M. Salciccioli, S. M. Edie and D. Vlachos, J. Phys. Chem. C, 2012, 116, 1873–1886 CAS.
- Q. Q. Luo, T. Wang, M. Beller and H. J. Jiao, J. Mol. Catal. A: Chem., 2013, 379, 169–177 CrossRef CAS PubMed.
- Y. Y. Wang, Y. Y. Qi, D. J. Zhang and C. B. Liu, J. Phys. Chem. C, 2014, 118, 2067–2076 CAS.
- S. Park, Y. Xie and M. J. Weaver, Langmuir, 2002, 18, 5792–5798 CrossRef CAS.
- D. Volpe, E. Casado-Rivera, L. Alden, C. Lind, K. Hagerdon, C. Downie, C. Korzeniewski, F. J. DiSalvo and H. D. Abruna, J. Electrochem.Soc., 2004, 151, A971–A977 CrossRef CAS PubMed.
- L. C. Grabow, A. A. Gokhale, S. T. Evans, J. A. Dumesic and M. Mavrikakis, J. Phys. Chem. C, 2008, 112, 4608–4617 CAS.
- Q. Q. Luo, G. Feng, M. Beller and H. J. Jiao, J. Phys. Chem. C, 2012, 116, 4149–4156 CAS.
- J. Wang, H. Zhang, K. Jiang and W. Cai, J. Am. Chem. Soc., 2011, 133, 14876–14879 CrossRef CAS PubMed.
- N. R. Avery, B. H. Toby, A. B. Anton and W. H. Weinberg, Surf. Sci. Lett., 1982, 122, L574–L578 CrossRef CAS.
- L. A. Larson and J. T. Dickinson, Surf. Sci., 1979, 84, 17–30 CrossRef CAS.
- W. Zhong, Y. Liu and D. Zhang, J. Phys. Chem. C, 2012, 116, 2994–3000 CAS.
- W. Zhong, R. Wang, D. Zhang and C. Liu, J. Phys. Chem. C, 2012, 116, 24143–24150 CAS.
- C. Taylor, R. G. Kelly and M. Neurock, J. Electrochem. Soc., 2006, 153, E207–E214 CrossRef CAS PubMed.
- K. Kunimatsu and H. Kita, J. Electroanal. Chem. Interfacial Electrochem., 1987, 218, 155–172 CrossRef CAS.
- D. S. Corrigan and M. J. Weaver, J. Electroanal. Chem. Interfacial Electrochem., 1988, 241, 143–162 CrossRef CAS.
- A. Miki, S. Ye and M. Osawa, Chem. Commun., 2002, 1500–1501 RSC.
- V. Grozovski, V. Climent, E. Herrero and J. M. Feliu, ChemPhysChem, 2009, 10, 1922–1926 CrossRef CAS PubMed.
- J. H. Choi, K. J. Jeong, Y. Dong, J. Han, T. H. Lim, J. S. Lee and Y. E. Sung, J. Power Sources, 2006, 163, 71–75 CrossRef CAS PubMed.
- C. X. Xu, Y. Zhang, L. Q. Wang, L. Q. Xu, X. F. Bian, H. Y. Ma and Y. Ding, Chem. Mater., 2009, 21, 3110–3116 CrossRef CAS.
- R. Mu, Q. Fu, H. Xu, H. Zhang, Y. Huang, Z. Jiang, S. Zhang, D. Tan and X. Bao, J. Am. Chem. Soc., 2011, 133, 1978–1986 CrossRef CAS PubMed.
- S. Kang, J. Lee, J. K. Lee, S. Y. Chung and Y. Tak, J. Phys. Chem. B, 2006, 110, 7270–7274 CrossRef CAS PubMed.
- S. Uhm, S. T. Chung and J. Lee, Electrochem. Commun., 2007, 9, 2027–2031 CrossRef CAS PubMed.
- N. Kristian, Y. Yan and X. Wang, Chem. Commun., 2008, 353–355 RSC.
- J. B. Xu, T. S. Zhao, Z. X. Liang and L. D. Zhu, Chem. Mater., 2008, 20, 1688–1690 CrossRef CAS.
- B. Peng, H. F. Wang, Z. P. Liu and W. B. Cai, J. Phys. Chem. C, 2010, 114, 3102–3107 CAS.
- J. Liu, L. Cao, W. Huang and Z. Li, ACS Appl. Mater. Interfaces, 2011, 3, 3552–3558 CAS.
- B. W. Zhang, C. L. He, Y. X. Jiang, M. H. Chen, Y. Y. Li, L. Rao and S. G. Sun, Electrochem. Commun., 2012, 25, 105–108 CrossRef CAS PubMed.
- Y. J. Kang, L. Qi, M. Li, R. E. Diaz, D. Su, R. R. Adzic, E. Stach, J. Li and C. B. Murray, ACS Nano, 2012, 6, 2818–2825 CrossRef CAS PubMed.
- U. B. Demirci, J. Power Sources, 2007, 173, 11–18 CrossRef CAS PubMed.
- K. Jiang, H. X. Zhang, S. Z. Zou and W. B. Cai, Phys. Chem. Chem. Phys., 2014, 16, 20360–20376 RSC.
- B. Hammer and J. K. Nørskov, in Adv. Catal., ed. H. K. Bruce and C. Gates, Academic Press, 2000, vol. 45, pp. 71–129 Search PubMed.
- J. Kitchin, J. K. Nørskov, M. Barteau and J. Chen, J. Chem. Phys., 2004, 120, 10240–10246 CrossRef CAS PubMed.
- N. M. Marković, H. A. Gasteiger, P. N. Ross Jr, X. Jiang, I. Villegas and M. J. Weaver, Electrochim. Acta, 1995, 40, 91–98 CrossRef.
- M. A. Rigsby, W. P. Zhou, A. Lewera, H. T. Duong, P. S. Bagus, W. Jaegermann, R. Hunger and A. Wieckowski, J. Phys. Chem. C, 2008, 112, 15595–15601 CAS.
- M. Arenz, V. Stamenkovic, T. J. Schmidt, K. Wandelt, P. N. Ross and N. M. Markovic, Phys. Chem. Chem. Phys., 2003, 5, 4242–4251 RSC.
- W. Chen, J. Kim, S. Sun and S. Chen, Langmuir, 2007, 23, 11303–11310 CrossRef CAS PubMed.
- C. Hu, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, Chem. Commun., 2012, 48, 11865–11867 RSC.
- A. López-Cudero, F. J. Vidal-Iglesias, J. Solla-Gullón, E. Herrero, A. Aldaz and J. M. Feliu, J. Electroanal. Chem., 2009, 637, 63–71 CrossRef PubMed.
- B. J. Hwang, S. M. Senthil Kumar, C. H. Chen, R. W. Chang, D. G. Liu and J. F. Lee, J. Phys. Chem. C, 2008, 112, 2370–2377 CAS.
- J. B. Xu, T. S. Zhao and Z. X. Liang, J. Phys. Chem. C, 2008, 112, 17362–17367 CAS.
- M. D. Segall, J. D. L. Philip, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567–570 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- W. Gao, J. A. Keith, J. Anton and T. Jacob, Dalton Trans., 2010, 39, 8450–8456 RSC.
- W. Gao, J. A. Keith, J. Anton and T. Jacob, J. Am. Chem. Soc., 2010, 132, 18377–18385 CrossRef PubMed.
- G. Feng, D. Yang, D. Kong, J. Liu and Z.-H. Lu, RSC Adv., 2014, 4, 47906–47920 RSC.
- J. Liu, Z.-F. Liu, G. Feng and D. Kong, J. Phys. Chem. C, 2014, 118, 18496–18504 CAS.
- X. H. Xia and T. Iwasita, J. Electrochem. Soc., 1993, 140, 2559–2565 CrossRef CAS PubMed.
- J. Jiang and A. Kucernak, J. Electroanal. Chem., 2002, 520, 64–70 CrossRef CAS.
- Y. X. Chen, M. Heinen, Z. Jusys and R. J. Behm, Angew. Chem., Int. Ed., 2006, 45, 981–985 CrossRef CAS PubMed.
- Y. X. Chen, M. Heinen, Z. Jusys and R. J. Behm, Langmuir, 2006, 22, 10399–10408 CrossRef CAS PubMed.
- X. Yu and P. G. Pickup, J. Power Sources, 2008, 182, 124–132 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |