Basic polymerized imidazolide-based ionic liquid: an efficient catalyst for aqueous Knoevenagel condensation†
Libing Dinga,
Hansheng Lia,
Yaping Zhanga,
Kun Zhanga,
Hong Yuana,
Qin Wu*a,
Yun Zhaoa,
Qingze Jiaoab and
Daxin Shi*a
aSchool of Chemical Engineering and the Environment, Beijing Institute of Technology, Beijing 100081, China. E-mail: dinglibing13@sina.com; hanshengli@bit.edu.cn; luckynanbin@gmail.com; zhangkun19910912@163.com; yuanhong@bit.edu.cn; wuqin_bit@126.com; zhaoyun@bit.edu.cn; jiaoqz@bit.edu.cn; shidaxin@bit.edu.cn; Fax: +86-10-68918979; Tel: +86-10-68918979
bSchool of Chemical Engineering and Materials Science, Beijing Institute of Technology, Zhuhai, 519085, China
First published on 18th February 2015
Abstract
A novel basic polymerized ionic liquid (BPIL): polymeric 1-[(4-ethenylphenyl)methyl]-3-propylimidazolium imidazolide was synthesized and characterized by Fourier transform infrared (FT-IR), nuclear magnetic resonance (NMR) and electron spray ionization mass spectrometry (ESI-MS). The BPIL was used as an efficient catalyst for aqueous Knoevenagel condensations with extended substrates. In comparison with common base catalysts, the BPIL showed high catalytic activity, which was ascribed to the cooperation between the strong basicity and the high surface activity. Moreover, the BPIL with high molecular weight has high surface activity and low catalytic activity. In addition, the BPIL was easily recovered and maintained high catalytic activity after five cycles of use in the system using benzaldehyde and malononitrile as substrates.
Introduction
The Knoevenagel condensation is one of the most powerful strategies for carbon–carbon bond formation in organic chemistry,1 and its products have numerous applications in the synthesis of fine chemicals, as well as carbocyclic and heterocyclic compounds of biological significance.2,3 This reaction is generally carried out between a carbonyl group and active methylene compounds in organic solvents in the presence of bases, mainly including piperidine,4 pyridine,5 ammonia, amines6 or sodium ethoxide. Recently, some novel heterogeneous catalysts, such as Ni–SiO2-supported catalysts,7 nitrogen-doped carbon materials,8 have been exploited as catalysts for the Knoevenagel condensation. However, many of these procedures have some or one of the disadvantages, such as a large amount of catalysts and organic solvent, a long reaction time, harsh reaction conditions, cumbersome purification process, no catalyst recovery.9 In addition, the utilization of volatile, hazardous, carcinogenic or costly solvents, especially, ethanol and toluene, has limited the wide application of the reaction in industrial processes.10With the increasing public concern over environmental degradation, the use of aqueous Knoevenagel condensations represents very powerful greenchemical technology procedures from both the economical and synthetic point of view.11 In comparison with organic solvent, water is a desirable solvent for the reasons of cost, nontoxic, nonflammable, cheap and available.12 In addition, reactions in aqueous media illustrate unique reactivity and selectivity that are not usually observed in organic media.13,14 However, the major drawback of aqueous media is the limited solubility of various organic compounds in water to reducing reaction interface area.15 Efforts have been made to perform the Knoevenagel condensation in aqueous media, which is usually catalyzed by Lewis acids16 or bases, and requires drastic conditions.17 Some reactions are performed on solid supports, promoted by infrared, ultrasound or microwave.18,19
A possible way to improve the solubility of substrates is the use of surface-active agents that can form micelles in aqueous media.20 Micelles can improve the solubility of substrates through solubilization.21 The solubilization of reactants and products inside the micelles not only results in high concentration within the small volume, but the different orientations of the solubilization may also significantly influence the reaction mechanism, resulting in much different reaction rates.14,19 The use of micelle is widespread and has been investigated in detail for different reactions in aqueous solution.19 However, it has not been reported in Knoevenagel condensations.
Long-chained ionic liquids have been extensively studied in the field of colloid and interface science because of the close resemblance of the alkyls in these ionic liquids' molecules to the long hydrocarbon chains of conventional surfactant molecules.22 Polymerized ionic liquids (PILs) are synthesized by introducing polymerizable group into ionic liquids,23 which process the unique properties of ionic liquids and macromolecular architecture, such as negligible vapour pressure, good stability, easily shaping and variety of available structures.24 PILs are special ionic liquids. The micelles form in aqueous solutions and are good surfactant and novel catalysts. Aqueous solution of PILs with basicity and surface activity is a potential efficient, green, recoverable and low-cost catalytic system. However, it has not been reported in Knoevenagel condensations.
In this work, we carried out Knoevenagel condensation catalyzed by a novel basic polymerized ionic liquid (BPIL): polymeric 1-[(4-ethenylphenyl)methyl]-3-propylimidazolium imidazolide. The procedure was environmentally friendly and efficient, and the BPIL possessed high catalytic activity and could be easily separated.
Experimental section
Materials
1-propylimidazole was purchased from Energy-Chemical. 4-chloromethylstyrene was obtained from Tokyo Kasei Kogyo Co., Ltd. Imidazole was purchased from Linhai Kaile Chemical Factory. 2,2-azobisisobutyronitrile (AIBN) was obtained from Sinopharm Chemical Reagent Beijing Co., Ltd. Sodium hydroxide, methanol, ethanol, ethyl ether (Et2O), ethyl acetate (EtOAc) and dimethylformamide (DMF) were received from Beijing Chemical Co., Ltd. All reagents and starting materials were commercially available and used without any further purification unless, otherwise, noted.Synthesis of basic polymeric 1-[(4-ethenylphenyl)methyl]-3-propylimidazolium imidazolide (BPIL)
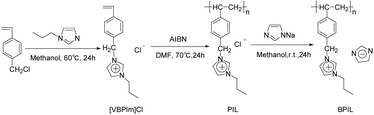
The synthetic procedure of BPIL was illustrated in Scheme 1. The 1-propylimidazole was dissolved in methanol and a stoichiometric amount of 4-chloromethylstyrene was added and stirred at 60 °C for 24 h, resulting in the formation of the 1-[(4-ethenylphenyl)methyl]-3-propylimidazolium chlorine [VBPIm]Cl, which was purified through removing methanol by rotary evaporation and washing with Et2O. [VBPIm]Cl exhibited excellent solubility in high polar solvents such as water, methanol and ethanol. [VBPIm]Cl monomer, AIBN and DMF were charged into a 100 mL three-neck flask. The flask, dried at high temperature, tightly sealed and purged with nitrogen gas, was immersed in an oil bath at 70 °C for 24 h for complete polymerization. The polymer (PIL) prepared was precipitated with ethyl acetate (EtOAc) and washed three times with EtOAc and Et2O to remove solvent. The final product was dried in a vacuum oven at 55 °C for 24 h to produce a powder.1.2 equivalents of imidazole, NaOH and deionized water were added into a three-neck flask, equipped with a condenser tube. The mixture was stirred at 95 °C for 4 h, resulting in the formation of sodium imidazole, then PIL methanol solution was added at room temperature and stirred for 24 h. Methanol was reduced by rotary evaporation and the mixture was washed with Et2O for three times to remove remaining imidazole. The mixture was dissolved in DMF and white NaCl was precipitated. The filtrate was poured into EtOAc, the BPIL was precipitated and placed in a freezer. The imidazolide anions content of BPIL was determined through hydrochloric acid titration and the exchange rate was deduced to be only 50%.
Methods
The structures of the ionic liquid polymer and its intermediates were analyzed by IR spectroscopy, NMR and electron spray ionization mass spectrometry (ESI-MS). IR spectra was recorded on a Perkin-Elmer Spectrum One FT-IR spectrometer using KBr windows suitable for Fourier transform infrared (FT-IR) transmittance technology to form a liquid film. 1H NMR spectra was obtained on a Varian Mercury-plus 400 nuclear magnetic resonance spectrometer. Molecular weight was recorded on a Varian 600 MS ESI-MS with a flow rate of the flow phase (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 2
methanol = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) for 200 μL min−1, capillary voltage of 80.0 volts, pinpoint voltage of 5000 volts and −5000 volts respectively under the positive and negative mode and the quality scanning range from 50 to 500 m/z. The component contents of mixture after Knoevenagel condensation was analyzed by Liquid Chromatogram (LC) with a flow rate of the mobile phase (methanol
3) for 200 μL min−1, capillary voltage of 80.0 volts, pinpoint voltage of 5000 volts and −5000 volts respectively under the positive and negative mode and the quality scanning range from 50 to 500 m/z. The component contents of mixture after Knoevenagel condensation was analyzed by Liquid Chromatogram (LC) with a flow rate of the mobile phase (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 9
water = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 0.3 mL s−1 at a wavelength of 256 nm.
1) for 0.3 mL s−1 at a wavelength of 256 nm.
Knoevenagel condensations catalyzed by BPIL
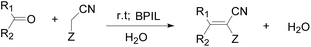
BPIL-catalyzed Knoevenagel condensation of a wide range of aldehydes and ketones with active methylene compounds leading to the formation of substituted electrophilic alkenes (Scheme 2). The typical process was carried out as follows. 50 mmol benzaldehyde and 50 mmol ethyl cyanoacetate or malononitrile were charged into a 25 mL round-bottomed flask equipped with a magnetic stirrer, followed by adding aqueous solution containing a certain amount of BPIL catalyst. The mixture was stirred at room temperature for 150 min. The progress was monitored by LC. After the completion of the reaction, the reaction mixture was poured into ice water. The solid product was precipitated and placed for several hours. The product was isolated by direct decantation and the catalyst was reused after the excessive water was removed.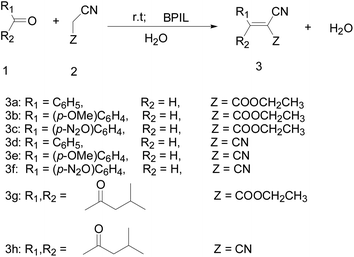 | ||
| Scheme 2 Knoevenagel condensation of aldehydes and ketones with active methylene compounds catalyzed by BPIL. | ||
The reaction process of 4-methoxybenzaldehyde and ethyl cyanoacetate or malononitrile was the same to that of benzaldehyde and ethyl cyanoacetate.
In the reaction of 4-nitrobenzaldehyde and ethyl cyanoacetate or malononitrile, yellow solid containing product and 4-nitrobenzaldehyde was precipitated and dissolved in EtOAc after filtrate. The product was purified though recrystallized from ethanol.
When the reaction of 3-methylbutanone and ethyl cyanoacetate or malononitrile was complete, the oily phase was extracted into EtOAc. After removing EtOAc, the product was obtained.
The products were analyzed by FT-IR and 1H NMR spectroscopy. The IR and 1H NMR spectral data were as follow:
Results and discussion
Characterization of the BPIL and its intermediates
The BPIL was characterized and confirmed by IR, NMR and ESI-MS. Fig. 1 showed the IR spectra of BPIL, together with those of [VBPIm]Cl and PIL. As shown in Fig. 1(a), for the [VBPIm]Cl, the![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretching vibrations was observed at the 3130 cm−1 and 3088 cm−1. The bands at 2968 cm−1 and 2877 cm−1 were assigned to the C–H vibration. The 1629 cm−1 was assigned to C
C–H stretching vibrations was observed at the 3130 cm−1 and 3088 cm−1. The bands at 2968 cm−1 and 2877 cm−1 were assigned to the C–H vibration. The 1629 cm−1 was assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C absorption peak, respectively. The vibration peaks of imidazole ring were observed at 1560 and 1452 cm−1. As can be seen from Fig. 1(b), for the PIL, the peak of C
C absorption peak, respectively. The vibration peaks of imidazole ring were observed at 1560 and 1452 cm−1. As can be seen from Fig. 1(b), for the PIL, the peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was disappeared. The formation of PIL was evident from the results conducted by IR spectra. In addition, a new band at 1663 cm−1 was assigned to C
C was disappeared. The formation of PIL was evident from the results conducted by IR spectra. In addition, a new band at 1663 cm−1 was assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in solvent. In comparison with PIL, BPIL showed obvious enhanced vibration peaks of imidazole ring, which showed the existence of imidazolide anion.
O stretching vibration in solvent. In comparison with PIL, BPIL showed obvious enhanced vibration peaks of imidazole ring, which showed the existence of imidazolide anion.
The BPIL and its intermediates were analyzed by 1H NMR spectroscopy and ESI-MS. The NMR spectral data were as follows. [VBPIm]Cl: 1H NMR (400 MHz, DMSO-d6): δ = 9.69 (1H, s), 7.92 (1H, s), 7.89 (1H, s), 7.51 (2H, d), 7.45 (2H, d), 6.73 (1H, m), 5.86 (1H, d), 5.49 (2H, s), 5.28 (1H, d), 4.16 (2H, t), 1.80 (2H, m), 0.83 ppm (3H, t). BPIL: 1H NMR (400 MHz, DMSO-d6): δ = 10.10–10.50 (1H, s), 7.85–8.20 (2H, br), 7.32–7.44 (4H, br), 5.30–5.78 (2H, br), 4.13–4.18 (3H, br), 1.72–1.76 (4H, br), 0.75–0.85 ppm (3H, br). The peak of 67 was observed under the negative ion mode, which also showed the existence of imidazolide anion.
The NMR and ESI-MS spectral data of BPIL and its ionic liquid intermediates agreed with their designed structures (Scheme 1).
A series of PILs with different molecular weight were prepared with different amount of AIBN as initiator and molecular weight of PIL was measured using Ubbelohde viscometer. As can be seen from Fig. 2, its molecular weight decreased sharply firstly, then reduced slowly and reached equilibrium finally as the amount of initiator increased.
The catalytic application of BPIL in aqueous Knoevenagel condensations
A preliminary study of the catalytic property of BPIL in the Knoevenagel condensation of benzaldehyde (BE) and ethyl cyanoacetate (EC) was carried out. As can be seen from Fig. 3(a), the product was formed within a few seconds in the presence of BPIL-3% (Amount of AIBN = 3 mol%), and the yield of the product increased when the reaction time was extended generally. The reaction had approached equilibrium when the reaction time was extended to about 150 minutes.Parallel experiments for amount of the catalyst under 150 min were carried out (Fig. 4). The results showed that 0.5 mol% alkaline moieties had excellent catalytic activity. The target compound was obtained with 99.2% isolated yield. Catalytic activity cites increased with the increase of catalyst amount, and reactants could interact with catalytic centers adequately, which caused higher catalytic activity.25
In order to demonstrate the superiority of BPIL as the catalyst, the contrast tests were conducted. As shown in Fig. 3(b), when NaOH, which was the well-known strong base, was used as the catalyst, it presented a low yield of 44.7% in Table 1. In contrast, BPIL-3% as the catalyst increased the yield up to 99.2% easily in a shorter reaction time (Fig. 3(b) and Table 1, entry 1). Under the same reaction condition, the catalytic activity of NaOH was obviously lower than that of BPIL-3%. Meanwhile, the catalytic activity of 1-benzyl-3-propyl-imidazolium imidazolide (BIL) with a similar structure to that of the monomer [VBPIm]Cl was also investigated. The result showed that the catalytic activity of BIL was lower than that of BPIL-3% and the yield of the target compound was 88.9% yield.
Surface activity was considered as an important factor that affected their catalytic activities. BPIL with a long alkyl chain processed high surface activity, but NaOH not. Aqueous Knoevenagel condensation occurred on the tiny liquid–liquid interface between reactants and water due to the insolubility of reactants in water. When NaOH was used as the catalyst, reactants could not interact with catalytic centers adequately on the reaction time scale, resulting in low catalytic activity and yield.
BPIL with a hydrophobic group showed high surface activity, so it was also a good new-type surfactant, which reduced the surface tension of aqueous solution and micelles formed in aqueous medium spontaneously.26 To further understand the superiority of superior surface activity of BPIL, critical micelle concentration (CMC) value of BPIL was investigated. CMC is of crucial importance for judging the micelle formation of surfactants. The measurement of electric conductivity was usually used for determining CMC of aqueous solution of surfactant.24 In this work, electroconductibility of different BPIL concentration of the aqueous solution were tested at room temperature (25 °C) and results for a typical measurement was shown in Fig. 5. CMC was determined by computing the second derivative of the local polynomial fit, and the minimum were recorded.27 As can be seen from Fig. 5(a), the CMC of BPIL-3% was deduced to be 178 mg L−1. For practical use and application, a surfactant with low CMC is often considered to play an efficient role in solubilization.28 Moreover, the CMC of BPIL-1%, BPIL-2% and BIL were deduced to be 33, 90 and 2533 mg L−1 (Fig. 5(b–d)).
When BPIL was used for the aqueous Knoevenagel condensation, organic substances molecules diffused to the aqueous–organic interface and their aqueous solubility increased via their incorporation into micelles in surfactants solution,29 which contributed to the interaction of reactants with catalytic centers. Consequently, BPIL showed higher catalytic activity than NaOH.
To confirm the above analysis, two parallel experiments with NaOH and cetyltrimethyl ammonium bromide (CTAB), as surfactant, were conducted, and the results were shown in Table 1. As can be seen from Table 1, when c(CTAB) was ten times more than its value of CMC,30,31 the yield was increased up to 73.3% (entry 4 in Table 1), and when c(CTAB) was up at 75 times, which was equal to that of BPIL-3% added, a high yield 89.7% was obtained (Table 1, entry 5).
In compared with BIL, BPIL exhibited higher catalytic activity because it had higher surface activity and its catalytic active centers were distributed around the main chain densely, improving the utilization rate of the ionic liquid.
Effect of molecular weight on catalytic activity was investigated. The results were summarized in Table 1. As can be seen from Table 1, when BPIL-2% was used as the catalyst, it presented a low yield of 68.8% (Table 1, entry 6) and BPIL-1% as the catalyst decreased the yield up to 60.1% (Table 1, entry 7). The solubility in the water has great effect on the catalytic activity of BPIL beside surface activity. The high the degree of polymerization of BPIL resulted in low solubility and poor extensibility in water, which led to BPIL aggregate. And catalytic centers were enwrapped in alkyl chain to some extent, which restricted the interaction of reactants with catalytic centers. As the degree of polymerization of BPIL increased, the yield of target product and catalytic activity of BPIL decreased.
With the optimal reaction system in hand, we then investigated the Knoevenagel condensation of various aromatic aldehydes/aliphatic ketones with malononitrile/ethyl cyanoacetate. It can be observed from Table 2 that two active methylene compounds could react smoothly with various aromatic aldehydes and aliphatic ketones. The effect of substituent at the aromatic ring on the Knoevenagel reaction was also studied (Table 2, entries 1–6). Generally, substrates with electron-withdrawing groups (such as nitro group) were more reactive than those with electron-donating groups (such as methoxy). In addition, Knoevenagel condensation of aliphatic ketones with active methylene ingredients also underwent smoothly at room temperature (Table 2, entries 7–10).
Finally, from the green chemistry point of view, recycling was a major concern. The Knoevenagel condensation of benzaldehyde and ethyl cyanoacetate was carried out (Fig. 6). An excellent yield of 99.2% for the condensational product was achieved. On the same scale, the recyclability of the catalytic system (S1) was investigated using the same reaction as model reaction. Upon the completion of the reaction, the reaction mixture was poured into ice water, the solid product was precipitated. The product was isolated by direct decantation and the catalyst was reused after the excessive water was removed by rotary evaporation at 50 °C. As shown in Fig. 6, the catalyst could be recycled three times and the yield of target product was about 90%. Another catalytic system (S2) using benzaldehyde and malononitrile as substrates was also investigated. After the reaction, the product was isolated via filtration and the filtrate containing catalyst was used in subsequent reactions directly. The catalyst could maintain high activity after five cycles of use. The analysis result showed that the trades of the catalyst adsorbed on the surface of the solid product in the filtration led to the loss of the catalyst, but deactivated due to high temperature treatment in the cycle would be the major fact.
Catalytic reaction process and mechanism of the aqueous Knoevenagel condensation of aromatic and aliphatic carbonyl compounds using BPIL were presented in Fig. 7. BPIL formed micelles because of intermolecular force in aqueous solution. Then, the micelles solution was added to reaction substrate, the reaction system formed two phases, then change into a system of oil in water (O/W) after rapid stirring. Benzaldehyde and ethyl cyanoacetate molecules diffused to the aqueous–organic interface easily due to low surface tension of aqueous solution and their concentrations in the surface of micelle phase were increased due to the solubilization of reactants, which favored the interaction of reactants with catalytic centers. Water produced in reaction was into the aqueous phase and organic products into the oil phase. After the completion of the reaction, the reaction mixture was poured into ice water, the solid product was precipitated and placed for several hours. The product was isolated by direct decantation, and the catalyst could be reused after the excessive water was removed.
 | ||
| Fig. 7 The process and mechanism of Knoevenagel condensation catalyzed by BPIL as catalyst and surfactant. | ||
Conclusion
In conclusion, we developed a novel polymerized ionic liquid (BPIL): polymeric 1-[(4-ethenylphenyl)methyl]-3-propylimidazolium imidazolide and it was characterized by IR, 1H NMR and ESI-MS. BPIL was used as efficient homogeneous catalysts for the aqueous Knoevenagel condensation reaction of various aromatic aldehyde/aliphatic ketone with active methylene compounds under room temperature. In comparison with NaOH catalyst, BPIL showed high catalytic activity due to intensive base sites and high surface activity. However, the BPIL with high molecular weight have high surface activity and low catalytic performance. The catalyst could be recovered and maintain high catalytic activity after five cycles of use in the system using benzaldehyde and malononitrile as substrates.Acknowledgements
This work is financially supported by the International Science & Technology Cooperation Program of China (no. 2012DFR40240), the National Nature Science Foundation of China (no. 20976013) and the Foundation of Beijing Key Laboratory for Chemical Power Source and Green Catalysis (no. 2014CX02026).Notes and references
- X. Z. Dong, Y. H. Hui, S. L. Xie, P. Zhang, G. P. Zhou and Z. F. Xie, RSC Adv., 2013, 3, 3222 RSC.
- Y. M. Ren and C. Cai, Catal. Lett., 2007, 118, 134 CrossRef CAS.
- M. L. Deb and P. J. Bhuyan, Tetrahedron Lett., 2005, 46, 6453 CrossRef CAS PubMed.
- C. Mukhopadhyay and A. Datta, Synth. Commun., 2008, 38, 2103 CrossRef CAS.
- F. Santamarta, P. Verdia and E. Tojo, Catal. Commun., 2008, 9, 1779 CAS.
- Z. J. Zheng, L. X. Liu, G. Gao, H. Dong, J. X. Jiang, G. Q. Lai and L. W. Xu, RSC Adv., 2012, 2, 2895 RSC.
- V. S. R. R. Pullabhotla, A. Rahman and S. B. Jonnalagadda, Catal. Commun., 2009, 10, 365 CrossRef CAS PubMed.
- N. Kannari, S. Okamura, S. Fujita, J. Ozaki and M. Arai, Adv. Synth. Catal., 2010, 352, 1476 CrossRef CAS.
- A. G. Ying, H. D. Liang, R. H. Zheng, C. H. Ge, H. J. Jiang and C. L. Wu, Res. Chem. Intermed., 2011, 37, 579 CrossRef CAS PubMed.
- Z. Chen, X. Dong, J. W. Wu and H. Xie, ISRN Org. Chem., 2011, 1 Search PubMed.
- X. L. Zhao, K. F. Yang, X. G. Liu, C. L. Ye, L. W. Xu and G. Q. lai, Aust. J. Chem., 2013, 66, 500 CrossRef CAS.
- U. M. Lindstrom, Organic Reaction in Water: Principles, Strategies and Application, Blackwell Publishing, 2007 Search PubMed.
- D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS.
- J. B. F. N. Engberts and M. Blandamer, Chem. Commun., 2001, 1701 RSC.
- J. J. Shrikhande, M. B. Gawande and R. V. Jayaram, Catal. Commun., 2008, 9, 1010 CrossRef CAS PubMed.
- A. J. Thakur, D. Prajapati, B. J. Gogoi and J. S. Sandhu, Chem. Lett., 2003, 32, 258 CrossRef CAS.
- F. Bigi, M. L. Conforti, R. Maggi, A. Piccinno and G. Sartori, Green Chem., 2000, 2, 101 RSC.
- M. L. Deb and P. J. Bhuyan, Tetrahedron Lett., 2005, 46, 6453 CrossRef CAS PubMed.
- B. Tamami and A. Fadavi, Iran. Polym. J., 2006, 15, 331 CAS.
- S. Sobhani and Z. P. Parizi, Tetrahedron, 2011, 67, 3540 CrossRef CAS PubMed.
- T. Dwars, E. Paetzold and G. Oehme, Angew. Chem., Int. Ed., 2005, 44, 7174 CrossRef CAS PubMed.
- A. L. Ping, P. P. Geng, J. H. Zhang, J. Liu, D. Z. Sun, X. D. Zhang, Q. X. Li, J. F. Liu and X. L. Wei, Soft Mater., 2014, 12, 326 CrossRef CAS.
- H. Zhang, Z. B. Zhou and J. Nie, Prog. Chem., 2013, 25, 761 CAS.
- J. K. Wang, Y. X. Zong, R. G. Fu, Y. Y. Niu, G. R. Yue, Z. J. Quan, X. C. Wang and Y. Pan, Ultrason. Sonochem., 2014, 21, 29 CrossRef CAS PubMed.
- X. W. Chen, X. H. Li and H. B. Song, Chin. J. Catal., 2008, 29, 957 CrossRef CAS.
- Z. G. Zhao, Chem. Ind. Press, 2006, 7 Search PubMed.
- C. Jungnickel, L. Justyna, R. Johannes, F. F. Jose, M. Anja and T. Jorg, Colloids Surf., A, 2008, 316, 278 CrossRef CAS PubMed.
- S. A. Zhou, L. Liu, B. Wang, M. G. Ma, F. Xu and R. C. Sun, Synth. Commun., 2010, 6, 1384 Search PubMed.
- H. Nakahara, Y. Kojima, Y. Moroi and O. Shibata, Langmuir, 2014, 30, 5771 CrossRef CAS PubMed.
- T. Yang, W. J. Li and C. H. Zhou, Petrochem. Technol. Appl., 2007, 25, 48 CAS.
- H. J. Lu, C. Chen, H. T. Guo, X. H. Zhou, J. F. Dong, X. L. Hong, X. F. Xi and G. Y. Zhang, Acta Chim. Sin., 2006, 64, 2437 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01700a |
| This journal is © The Royal Society of Chemistry 2015 |