A new family of energetic salts based on oxy-bridged bis(dinitromethyl)furazan: syntheses, characterization and properties†
Hui Li,
Feng-qi Zhao*,
Bo-zhou Wang,
Lian-jie Zhai,
Wei-peng Lai and
Ning Liu
Science and Technology on Combustion and Explosion Laboratory, Xi'an Modern Chemistry Research Institute, Xi'an, Shaanxi 710065, China. E-mail: npecc@163.com; Tel: +86-29-88291663
First published on 18th February 2015
Abstract
Energetic salts based on oxy-bridged bis(dinitromethyl)furazan (2) were synthesized and fully characterized by NMR (1H and 13C), IR spectroscopy, elemental analysis as well as differential scanning calorimetry (DSC). The crystal structures of neutral 2, its ammonium salt (4), guanidinium salt (7) and guanidylguanidinium salt (9) were also determined by single-crystal X-ray diffraction. Except for hydroxylammonium salt (5), all the remaining salts exhibit good thermal stabilities with decomposition temperature above 180 °C. Furthermore, the densities of salts ranged from 1.65 g cm−3 to 1.88 g cm−3. Theoretical calculations provided detonation pressures and velocities for the energetic salts within the range of 24.9–38.0 GPa and 7582.2–9072.7 m s−1, respectively.
Introduction
The syntheses of energetic materials with high energy and low sensitivity have attracted great interest all over the world.1 Traditional strategy for designing energetic compound is incorporating oxidizer component and fuel component in the same molecule, such as TNT (2,4,6-trinitrotoluene) and RDX (1,3,5-trinitro-1,3,5-triazacyclohexane), whose energy relied on the combustion of the carbon backbone while consuming the oxygen provided by the nitro groups.2 Oxygen balance (the index of the deficiency or excess of oxygen in a compound required to convert all carbon into carbon dioxide, and all hydrogen into water) plays an important role to enhance the detonation properties of energetic compound, however the improved oxygen balance usually result in the decreased thermal and impact stability.Unlike the traditional energetic materials, the energy of nitrogen-rich energetic materials originate from their high heat of formation due to their high N–N, C–N and N–O bonds contents.3 Of these, energetic salts with nitrogen-rich cations are one of the special classes of materials with higher performance and good stability toward heat and stimulus which are attributed to the substantial intra- and intermolecular hydrogen bonds.4 The syntheses of energetic salts are usually performed by the simple neutralization or metathesis reactions, thus leading to more opportunities to generate energetic material with high energy and low vulnerability characteristics. Most energetic salts consist of N, O or C deprotonated anions, including azolate N-anions,5 nitramide N-anions,6 oxide O-anions7 and dinitromethyl C-anions.8
Actually, the performances of energetic compounds are closely related to their density, oxygen balance and heat of formation.1 Other than higher heat of formation, higher oxygen content is also required for a molecule to possess improved properties. The incorporation of high content of oxygen and nitrogen into a compound is a new strategy to develop more powerful, less sensitivity and eco-friendly energetic materials. Recently, several nitrogen-containing heterocyclic anions with high percentage of oxygen have been designed and exhibit important role to enhance the performance of energetic salts, examples including 5-nitro-3-trinitromethyl-1H-1,2,4-triazole,9 5,5′-bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole,9 3,3′-dinitroamino-4,4′-azoxyfurazan10 and 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazole)11 based salts (Fig. 1).
Furazan compounds in combination with dinitromethyl substituents will be of particular interest since these compounds have satisfactory oxygen content. Based on the above consideration, oxy-bridged bis(dinitromethyl)furazan anion, a new nitrogen-containing heterocyclic anion with high percentage of oxygen, was designed to generate corresponding salts. Herein, the syntheses, characterization and properties of oxy-bridged bis(dinitromethyl)furazan based salts were reported, which have potential applications in solid propellants, explosives and pyrotechnics.
Results and discussion
Syntheses
Dipotassium oxy-bridged bis(dinitromethyl)furazan (1) was synthesized through a procedure described by our group (Scheme 1).12 Subsequently, oxy-bridged bis(dinitromethyl)furazan (2) was obtained by the treatment of 1 with 50% sulfuric acid. As for the syntheses of 3–9, the reactions of 1 with corresponding halide salts in water were tried firstly, but the attempts failed except for 3. Then, the neutralization of 2 with free bases in methanol solution was applied to generate 4–9 (Scheme 2).All the salts were stable in air and could be stored for days. Their structures were fully investigated and confirmed by 1H NMR, 13C NMR, IR spectroscopy and elemental analyses. It is notable that all the anions have similar chemical shifts in 13C NMR spectra (around 161.2, 142.8 and 119.1 ppm), indicating that the anions in the salts exist isolated in the solution. The 13C NMR signals of dinitromethyl group appear at higher field compared to the ones in literature (129 to 132 ppm),8a–g which resulted from the conjugation of negative charge throughout the aromatic rings.
X-ray crystallography
2 crystallizes in the monoclinic space group Cc and has a density of 1.840 g cm−3. As shown in Fig. 2, there are two crystallographic independent molecules in the asymmetric unit. In each molecule, the bridged oxygen atom is nearly coplanar with each furazan ring (torsion angle O6–C4–C5–N6 179.710°, O6–C3–C2–N3 179.326°, O17–C10–C11–N11 177.170°, O17–C9–C8–N14 178.607°). The dihedral angles of the two furazan rings are 8.674° and 8.727°, respectively. The existence of non-classical intermolecular hydrogen bonds and O⋯O week interactions contribute to an increase in density. | ||
| Fig. 2 (a) Molecular structure of 2 with thermal ellipsoids at 30% probability; (b) packing diagram of 2 viewed down the c axis. | ||
4 and 7 both crystallize in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and 9 crystallizes in the orthorhombic space group Pbcn. 4, 7 and 9 exhibit densities of 1.843, 1.765 and 1.678 g cm−3, respectively. The transfer of protons from dinitromethyl groups to ammonium, guanidine and guanidylguanidinium were confirmed, respectively. The structure of anions in different salts are similar with that of 2, the dihedral angles between two furazan rings (16.25°, 16.76° and 15.17°) are slightly bigger than 2. The lengths of C–C bonds connecting dinitromethyl group and furazan ring are slightly shorter than 2, which indicate the existing of delocalized π-electron system in the anion. In contrast to the previously reported 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazole) anion,11 the dinitromethyl groups and furazan rings on the same side of the bridged oxygen atom are clearly twisted rather than nearly planar, which contribute to the lower density than that of 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazole) based salts. As for the cations, guanidium cations in 7 crystallize in one plane, and guanidylguanidinium cations in 9 have a twist at N7 with dihedral angles of 41.7° between the two guanidinium planes. The discrete cations and anions are linked into 3D network by the extensive hydrogen-bonding interactions between cations and anions (Fig. 3, 4 and 5). The details of all hydrogen bonds are gathered in the ESI.†
, and 9 crystallizes in the orthorhombic space group Pbcn. 4, 7 and 9 exhibit densities of 1.843, 1.765 and 1.678 g cm−3, respectively. The transfer of protons from dinitromethyl groups to ammonium, guanidine and guanidylguanidinium were confirmed, respectively. The structure of anions in different salts are similar with that of 2, the dihedral angles between two furazan rings (16.25°, 16.76° and 15.17°) are slightly bigger than 2. The lengths of C–C bonds connecting dinitromethyl group and furazan ring are slightly shorter than 2, which indicate the existing of delocalized π-electron system in the anion. In contrast to the previously reported 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazole) anion,11 the dinitromethyl groups and furazan rings on the same side of the bridged oxygen atom are clearly twisted rather than nearly planar, which contribute to the lower density than that of 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazole) based salts. As for the cations, guanidium cations in 7 crystallize in one plane, and guanidylguanidinium cations in 9 have a twist at N7 with dihedral angles of 41.7° between the two guanidinium planes. The discrete cations and anions are linked into 3D network by the extensive hydrogen-bonding interactions between cations and anions (Fig. 3, 4 and 5). The details of all hydrogen bonds are gathered in the ESI.†
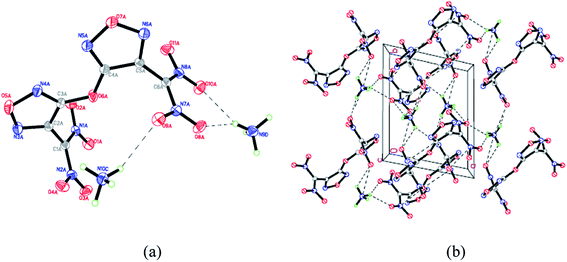 | ||
| Fig. 3 (a) Molecular structure of 4 with thermal ellipsoids at 30% probability; (b) packing diagram of 4 viewed down the c axis. | ||
 | ||
| Fig. 4 (a) Molecular structure of 7 with thermal ellipsoids at 30% probability; (b) packing diagram of 7 viewed down the b axis. | ||
 | ||
| Fig. 5 (a) Molecular structure of 9 with thermal ellipsoids at 30% probability; (b) packing diagram of 9 viewed down the c axis. | ||
Thermal stabilities and sensitivities
The thermal stabilities of 2–9 were investigated by DSC (10 °C min−1). As shown in Table 2, 2, 8 and 9 exhibit melting point of 117.0, 116.2 and 157.6 °C, respectively. 9 exhibits the highest decomposition temperature of 221.1 °C, while 5 shows the lowest decomposition temperature of 131.5 °C. All the other salts have decomposition temperature at range of 188.3 °C (4) to 199.4 °C (6).| No. | 2 | 4 | 7 | 9 |
|---|---|---|---|---|
| Empirical formula | C12H4N16O22 | C6H8N10O11 | C8H12N14O11 | C10H16N18O11 |
| Formula weight | 724.30 | 396.22 | 480.32 | 564.12 |
| Crystal system | Monoclinic | Triclinic | Triclinic | Orthorhombic |
| Space group | Cc | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Pbcn |
| a/nm | 1.4974(3) | 0.7691(4) | 0.8882(16) | 1.3873(9) |
| b/nm | 1.4968(3) | 0.9290(5) | 0.1025(18) | 1.1275(7) |
| c/nm | 1.1822(2) | 1.0564(5) | 0.1072(19) | 1.4334(10) |
| α/(°) | 90 | 94.504(8) | 78.079(3) | 90 |
| β/(°) | 99.260(3) | 104.174(7) | 83.683(3) | 90 |
| γ/(°) | 90 | 100.366(7) | 71.346(2) | 90 |
| V/nm3 | 2.6151(9) | 0.7139(6) | 0.9037(3) | 2.242(3) |
| Z | 4 | 2 | 2 | 8 |
| ρcalc/(g cm−3) | 1.840 | 1.843 | 1.765 | 1.678 |
| μ/mm−1 | 0.179 | 0.176 | 0.162 | 0.149 |
| F(000) | 1456 | 404 | 492 | 1168 |
| Goodness-of-fit on F2 | 1.035 | 1.081 | 1.040 | 1.042 |
| Final R indices [I > 2σ(I)] | R1 = 0.0478 | R1 = 0.0366 | R1 = 0.0401 | R1 = 0.0638 |
| wR2 = 0.1295 | wR2 = 0.1266 | wR2 = 0.1108 | wR2 = 0.1310 | |
| R indices (all data) | R1 = 0.0549 | R1 = 0.0410 | R1 = 0.0442 | R1 = 0.0948 |
| wR2 = 0.1364 | wR2 = 0.1382 | wR2 = 0.1153 | wR2 = 0.1702 | |
| No. | Tma (°C) | Tdb (°C) | ρc (g cm−3) | ΔfHcationd (kJ mol−1) | ΔfHanione (kJ mol−1) | ΔfHsaltf (kJ mol−1) | Ωg (%) | Ph (GPa) | Di (m s−1) | ISj (J) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Melting point.b Decomposition temperature.c Density measured by gas pycnometer (25 °C).d Calculated enthalpy of formation of cations.e Calculated enthalpy of formation of anions.f Calculated enthalpy of formation of salts.g Oxygen balance for CaHbOcNd, 1600(c − 2a − b/2)/Mw; Mw = molecular weight.h Detonation pressure (calculated with K–J equation).i Detonation velocity (calculated with K–J equation).j Impact sensitivity.k Crystal densities at 298 K.l From ref. 17a.m From ref. 17b. | ||||||||||
| 2 | 117.0 | 196.0 | 1.83(1.84)k | — | — | 167 | −8.8 | 34.8 | 8715.3 | 2 |
| 3 | — | 189.3 | 1.81 | 350.6l | −11.3 | −372.9 | −24.0 | 27.8 | 7828.9 | 15 |
| 4 | — | 188.3 | 1.81(1.84)k | 626.4l | −11.3 | 30.0 | −12.1 | 34.9 | 8719.7 | 5 |
| 5 | — | 131.5 | 1.85 | 669.5l | −11.3 | 135.0 | −5.6 | 37.8 | 9072.7 | 4.5 |
| 6 | — | 199.4 | 1.88 | 770.0l | −11.3 | 327.1 | −15.0 | 38.0 | 9049.6 | 7.5 |
| 7 | — | 186.6 | 1.74(1.77)k | 575.9l | −11.3 | 25.3 | −28.3 | 29.0 | 8055.2 | 32.5 |
| 8 | 116.2 | 191.6 | 1.65 | 875.1l | −11.3 | 715.9 | −25.3 | 27.7 | 8045.8 | 4 |
| 9 | 157.6 | 221.1 | 1.69(1.68)k | 620.9m | −11.3 | 192.9 | −38.3 | 24.9 | 7582.2 | 21 |
| RDX | — | 205 | 1.82 | — | — | 80.0 | −21.6 | 34.9 | 8748 | 7.4 |
The impact sensitivities for 2–9 were determined according to the standard BAM methods13 and the results were displayed in Table 2. Neutral compound (2) shows the highest sensitivity toward impact and is classified as “very sensitive”. All of the salts exhibit sensitivities between 4 J and 32.5 J and are classified as “sensitive”.14 Of these, 3, 7 and 9 are less sensitive than RDX (7.5 J)10 and 6 possesses impact sensitivity similar with RDX. Considering that the introduction of nitrogen-rich cations increase intra- and intermolecular hydrogen bonds, it is no surprise that 3–9 all are less sensitive (impact) than neutral compound (2).
Detonation parameters
The densities of all the salts were measured by a gas pycnometer (Table 2), falling in the range of 1.65 (8)–1.88 (6) g cm−3. The heats of formation, as one of the most important characteristics for energetic salts, were computed theoretically using the Gaussian 09 program package.15 The calculated standard enthalpy of formation for the anion was −11.3 kJ mol−1, a value higher than that of 5,5′-dinitromethyl-3,3′-bis(1,2,4-oxadiazole) anion (−101.7 kJ mol−1).11 Except for 3, the remaining salts were calculated to exhibit positive heat of formation. Among them, 8 possesses the highest heat of formation. The oxygen balances of all the salts range from −38.3% to −5.6%. Of the compounds, 4, 5 and 6 have oxygen balances of −24.0%, −12.1% and −5.6%, respectively, and thus are superior to RDX.With the calculated heats of formation and experimental densities, detonation parameters were calculated by the Kamlet–Jacobs equation.17 The calculated detonation pressures and velocities fall in the range of 24.9–38.0 GPa and 7582.2–9072.7 m s−1, respectively. In terms of detonation velocity, 5 and 6 even exceed the benchmark explosive RDX (8748 m s−1).8 Unfortunately, 5 have unfavorable thermal stability (Tdec: 131.5 °C) and impact sensitivity (greater than that of RDX).
Conclusion
A series of energetic salts with acceptable oxygen balance have been synthesized in moderate to excellent yields. Based on the fact that π-electron system is delocalized in the anion, all the salts exhibit good thermal stability with the decomposition temperatures ranged from 188.3 to 221.1 °C, with the exception of 5. Impact sensitivities of synthesized salts fall in the range from 4 to 32.5 J. Based on the experimental densities and calculated heat of formation, detonation pressures and velocities were calculated. Most of the salts have detonation velocities higher than 8000 m s−1 (except for 3 and 9), and some salts even exceed the conventional explosives RDX. Among them, hydrazidinium salt (6) exhibits promising comprehensive properties, such as comparable thermal stability and sensitivity with RDX, higher density and detonation property than RDX, make it a competitive secondary explosive.Experimental section
1H NMR and 13C NMR were obtained on a Bruker AV500 NMR spectrometer. Infrared spectra were obtained from KBr pellets on a Nicolet NEXUS870 Infrared spectrometer in the range of 4000–400 cm−1. Elemental analyses (C, H and N) were performed on a VARI-El-3 elemental analyzer. The DSC curves under a flowing nitrogen gas were obtained by a NETZSCH DSC200 F3 apparatus. Densities were measured at room temperature using a Micromeritics Accupyc II 1340 gas pycnometer. The impact sensitivities were determined on a ZBL-B impact sensitivity instrument (Nachen Co., China) with approximately 10 mg of sample.X-ray crystallography
Crystals of 2 was obtained by slow evaporation from the solution in CHCl3 at room temperature. Crystals of 4, 7 and 9 were obtained by slow evaporation from corresponding solution in water at room temperature. For all compounds, a Bruker SMART APE II CCD X-ray diffractometer was employed for data collection using Mo Kα radiation (k = 0.71073 Å). The structure was solved by direct methods using SHELXS program of the SHELXL-97 package and refined with SHELXL package.16 The final refinement was performed by full-matrix least-squares method with anisotropic thermal parameters on F2 for the non-hydrogen atoms. Crystal data and refinement results are summarized in Table 1.Theoretical study—computational details
Computations were performed with the Gaussian 09 (Revision B. 01) suite of programs.15 The geometric optimization of the structures and frequency analyses were carried out using B3LYP functional with the 6-31+G** basis set, and single-point energies were calculated at the MP2(full)/6-311++G** level. All of the optimized structures were characterized to be true local energy minima on the potential-energy surface without imaginary frequencies.Based on a Born–Haber energy cycle (Scheme 3), the standard heats of formation of a salt can be simplified by eqn (1):
| ΔH0f (salt, 298 K) = ΔH0f (cation, 298 K) + ΔH0f (anion, 298 K) − ΔHL | (1) |
| ΔHL = UPOT + [p(nM/2 − 2) + q(nx/2 − 2)]RT | (2) |
| UPOT = γ(ρm/Mm)1/3 + δ | (3) |
The gas-phase heat of formation for 2 was carried out by the application of isodesmic reaction shown in Scheme 4. The solid state enthalpy of formation of 2 was calculated by subtracting the enthalpy of sublimation, obtained by Trouton's rule (ΔHsub = 188 × Tm), from the gas-phase enthalpy.19
The detonation velocity (D) and detonation pressure (P) were evaluated by the empirical Kamlet–Jacobs (K–J) equations as shown in eqn (4)–(6).20
| P = 1.558ρ2Φ | (4) |
| D = 1.01Φ1/2(1.011 + 1.312ρ) | (5) |
| Φ = 0.4889N(MQ)1/2 | (6) |
D is the predicted detonation velocity (m s−1), P is the detonation pressure (GPa), and ρ is the compound density (g cm−3). Φ, N, M and Q are characteristic parameters of an explosive; Q is the chemical energy of detonation (kJ g−1). The densities and the calculated heats of formation were used to compute the D and P values.
Dipotassium oxy-bridged bis (dinitromethyl) furazan (1)
1 was prepared in a procedure described by our group.10 13C NMR (DMSO-d6, 125 MHz): δ = 160.8, 142.3, 118.7; IR (KBr, cm−1): 1479, 1239, 1070, 1589, 1526, 997; anal. calc. for C6N8O11K2: C, 16.44; N, 25.57. Found: C, 16.35; N, 25.36%.Oxy-bridged bis(dinitromethyl)furazan (2)
50% sulfuric acid (1 mL) was added to a solution of 1 (0.438 g, 1 mmol) in water (3 mL). After stirring for 10 min at room temperature, the reaction mixture was extracted with ethyl ether (3 × 10 mL). The extracts were washed with water and dried over magnesium sulfate. The ethyl ether solution was evaporated to obtain a white solid (0.295 g, 81.5%). 1H NMR (DMSO-d6, 500 MHz): δ = 10.49 (s, 2H, CH); 13C NMR (DMSO-d6, 125 MHz): δ = 161.2, 142.8, 119.1; IR (KBr, cm−1): ν = 3004, 2983, 1481, 1366, 1623, 1322, 1239, 1148, 1037, 1585, 1524, 999; calc. for C6H2N8O11: C 19.90, H 0.56, N 30.94%; found: C 19.84, H 0.52, N 30.78%.Bis(N-carbamoylguanidinium) oxy-bridged bis(dinitromethyl)furazan (3)
A mixture of N-carbamoylguanidinium chloride (0.250 g, 2 mmol) and 1 (0.438 g, 1 mmol) in water (10 mL) was stirred at 60 °C for 1 h. After the mixture was cooled to room temperature, the precipitate was filtered to give a yellow powder (0.363 g, 64.1%). 1H NMR (DMSO-d6, 500 MHz): δ = 9.60 (s, 2H, NH), 8.03 (br, 8H, NH2), 7.02 (br, 4H, NH2) ppm. 13C NMR (DMSO-d6, 125 MHz): δ = 161.2, 155.8, 154.8, 142.8, 119.1. Elemental anal. calc. for C10H14N16O13: C, 21.21; H, 2.49; N, 39.57; found: C, 21.15; H, 2.52; N, 39.47. IR (KBr, cm−1): ν = 3428, 3199, 1745, 1693, 1594, 1536, 1485, 1339, 1246, 1141, 998, 864, 824, 746.General procedure for synthesis of the salts 4–9
2 (1 mmol) was added to a solution of ammonia (2 mmol), hydrazine (2 mmol), hydroxylamine (2 mmol), guanidine (2 mmol), triaminoguanidine (2 mmol) and guanidylguanidine (2 mmol) in MeOH (10 mL), respectively. The resulting mixture stirred at room temperature overnight. The solution was slowly evaporated and the remaining solid was collected and washed with cool ethanol to give the desired products.Diammonium oxy-bridged bis(dinitromethyl)furazan (4)
Light yellow solid (255 mg, 64.4%). 1H NMR (DMSO-d6, 500 MHz): δ = 7.07 (t, J = 50.9 Hz, 8H, NH4) ppm. 13C NMR (DMSO-d6, 125 MHz): δ = 161.2, 142.8, 119.1 ppm. Elemental anal. calc. for C6H8N10O11: C, 18.19; H, 2.04; N, 35.35; found: C, 18.22; H, 2.01; N, 35.26; IR (KBr, cm−1): ν = 3211, 1599, 1578, 1536, 1496, 1430, 1274, 1140, 1074, 1000, 876, 818, 750.Dihydroxylammonium oxy-bridged bis(dinitromethyl)furazan (5)
Light yellow solid (310 mg, 72.4%). 1H NMR (DMSO-d6, 500 MHz): δ = 10.09 (s, 6H, NH3), 9.91 (s, 2H, OH) ppm. 13C NMR (DMSO-d6, 125 MHz): δ = 160.7, 142.2, 118.6 ppm. Elemental anal. calc. for C6H8N10O13: C, 16.83; H, 1.88; N, 32.71; found: C, 16.80; H, 1.92; N, 32.62. IR (KBr, cm−1): ν = 3424, 3150, 1578, 1534, 1483, 1329, 1305, 1239, 1148, 1070, 1000, 875, 819, 749.Dihydrazidinium oxy-bridged bis(dinitromethyl)furazan (6)
White solid (228 mg, 53.5%). 1H NMR (CD3OD, 500 MHz): δ = 4.95 (br, 10H, NH2, NH3) ppm. 13C NMR (CD3OD, 125 MHz): δ = 160.8, 142.3, 118.6 ppm. Elemental anal. calc. for C6H10N12O11: C, 16.91; H, 2.36; N, 39.44; found: C, 16.96; H, 2.32; N, 39.34; IR (KBr, cm−1): ν = 3443, 3329, 3148, 1582, 1536, 1481, 1384, 1324, 1238, 1144, 1072, 998, 877, 825, 743.Bis(guanidnium) 3,3′-bis(dinitromethyl)difurazanyl ether (7)
Yellow solid (418 mg, 87.1%). 1H NMR (DMSO-d6, 500 MHz): δ = 6.89 (s, 12H, NH2) ppm. 13C NMR (DMSO-d6, 125 MHz): δ = 161.2, 158.3, 142.8, 119.1 ppm. Elemental anal. calc. for C8H12N14O11: C, 20.01; H, 2.52; N, 40.83; found: C, 20.08; H, 2.49; N, 40.69. IR (KBr, cm−1): ν = 3423, 3202, 1662, 1538, 1468, 1387, 1326, 1238, 1142, 1074, 997, 866, 825, 747.Bis(triaminoguanidnium) oxy-bridged bis(dinitromethyl)furazan (8)
Yellow solid (355 mg, 62.3%). 1H NMR (DMSO-d6, 500 MHz): δ = 8.59 (s, 6H, NH), 4.49 (s, 12H, NH2) ppm. 13C NMR (DMSO-d6, 125 MHz): δ = 161.2, 159.5, 142.8, 119.1 ppm. Elemental anal. calc. for C8H18N20O11: C, 16.85; H, 3.18; N, 49.12; found: C, 16.90; H, 3.15; N, 48.97. IR (KBr, cm−1): ν = 3359, 3320, 3211, 1686, 1535, 1472, 1382, 1320, 1239, 1129, 1070, 999, 948, 884, 823, 748.Bis(guanidylguanidinium) oxy-bridged bis(dinitromethyl)furazan (9)
Yellow solid (412 mg, 89.2%). 1H NMR (DMSO-d6, 500 MHz): δ = 6.89 (s, 9H, NH and NH2); 13C NMR (DMSO-d6, 125 MHz): δ = 161.2, 159.9, 142.8, 119.1; elemental anal. calc. for C10H16N18O11: C, 21.28; H, 2.86; N, 44.67; found: C, 21.30; H, 2.78; N, 44.56; IR (KBr, cm−1): ν = 3457, 3357, 1620, 1562, 1520, 1464, 1235, 1144, 1057, 996, 883, 745.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21373157 and 21173163). Dr Yan-Jing Yang (Xi'an Modern Chemistry Research Institute, China) is acknowledged for his support on the language of the manuscript and valuable suggestions.References
- H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- A. A. Dippold and T. M. Klapötke, J. Am. Chem. Soc., 2013, 135, 9931–9938 CrossRef CAS PubMed.
- D. M. Badgujar, M. B. Talawar, S. N. Asthana and P. P. Mahulikar, J. Hazard. Mater., 2008, 151, 289–305 CrossRef CAS PubMed.
- J. Zhang, Q. Zhang, T. T. Vo, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2015, 137, 1697–1704 CrossRef CAS PubMed.
- (a) Y. Tang, H. Yang, J. Shen, B. Wu, X. Ju, C. Lu and G. Cheng, Eur. J. Inorg. Chem., 2014, 7, 1231–1238 CrossRef; (b) C. Bian, K. Wang, L. Liang, M. Zhang, C. Li and Z. Zhou, Eur. J. Inorg. Chem., 2014, 35, 6022–6030 CrossRef; (c) C. Li, L. Liang, K. Wang, C. Bian, J. Zhang and Z. Zhou, J. Mater. Chem. A, 2014, 2, 18097–18105 RSC; (d) V. Thottempudi, J. Zhang, C. He and J. M. Shreeve, RSC Adv., 2014, 4, 50361–50364 RSC.
- (a) M. A. Kettner, T. M. Klapötke, T. G. Müller and M. Suceska, Eur. J. Inorg. Chem., 2014, 28, 4756–4771 CrossRef; (b) D. Fischer, T. M. Klapötke, M. Reymann and J. Stierstorfer, Chem.–Eur. J., 2014, 20, 6401–6411 CrossRef CAS PubMed; (c) P. Yin, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2014, 53, 12889–12892 CrossRef CAS PubMed.
- (a) D. Fischer, T. M. Klapötke, M. Reymann, P. C. Schmid, J. Stierstorfer and M. Suceska, Propellants, Explos., Pyrotech., 2014, 39, 550–557 CrossRef CAS; (b) J.-T. Wu, J.-G. Zhang, X. Yin, P. He and T.-L. Zhang, Eur. J. Inorg. Chem., 2014, 27, 4690–4695 CrossRef; (c) J. Xu, J. Wei, F. Li, Q. Ma and X. Peng, New J. Chem., 2014, 38, 5303–5311 RSC.
- (a) H. Gao, Y.-H. Joo, D. A. Parrish, T. Vo and J. M. Shreeve, Chem.–Eur. J., 2011, 17, 4613–4618 CrossRef CAS PubMed; (b) J. Song, Z. Zhou, D. Cao, H. Huang, L. Liang, K. Wang and J. Zhang, Z. Anorg. Allg. Chem., 2012, 5, 811–820 CrossRef; (c) L. Liang, D. Cao, J. Song, H. Huang, K. Wang, C. Bian, X. Dong and Z. Zhou, J. Mater. Chem. A, 2013, 1, 8857–8865 RSC; (d) J. Song, Z. Zhou, X. Dong, H. Huang, D. Cao, L. Liang, K. Wang, J. Zhang, F. Chen and Y. Wu, J. Mater. Chem., 2012, 22, 3201–3209 RSC; (e) L. He, G. Tao, D. A. Parrish and J. M. Shreeve, Inorg. Chem., 2011, 50, 679–685 CrossRef CAS PubMed; (f) J. Song, Z. Zhou, H. Huang, D. Cao, L. Liang, K. Wang, J. Zhang and F. Zhao, Z. Anorg. Allg. Chem., 2012, 6, 957–964 CrossRef; (g) L. He, G. Tao, D. A. Parrish and J. M. Shreeve, Chem. Commun., 2013, 49, 10329–10331 RSC.
- V. Thottempudi and J. M. Shreeve, J. Am. Chem. Soc., 2011, 133, 19982–19992 CrossRef CAS PubMed.
- J. Zhang and J. M. Shreeve, J. Am. Chem. Soc., 2014, 136, 4437–4445 CrossRef CAS PubMed.
- T. M. Klapötke, N. Mayr, J. Stierstorfer and M. Weyrauther, Chem.–Eur. J., 2014, 20, 1410–1417 CrossRef PubMed.
- (a) L.-J. Zhai, B.-Z. Wang, X.-Z. Fan and X.-Z. Li, Chin. J. Struct. Chem., 2014, 33, 1353–1359 Search PubMed; (b) Y. Fan, B. Wang, W. Lai and P. Lian, Chin. J. Org. Chem., 2009, 29, 614–620 CAS.
- (a) http://www.bam.de; (b) NATO standardization agreement (STANAG) on Explosives, Impact Tests, no. 4489, 1st ed., Sept, 17, 1999.
- A range of impact sensitivities from UN recommendations: Insensitive, >40 J; less sensitive, ≥35 J; sensitive, ≥4 J; very sensitive, ≤3 J.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B. 01, Gaussian, Inc., Wallingford, CT, USA, 2009 Search PubMed.
- (a) G. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997 Search PubMed.
- (a) L. Liang, H. Huang, K. Wang, C. Bian, J. Song, L. Ling, F. Zhao and Z. Zhou, J. Mater. Chem., 2012, 22, 21954–21964 RSC; (b) R. Wang, Y. Guo, Z. Zeng, B. Twamley and J. M. Shreeve, Chem.–Eur. J., 2009, 15, 2625–2634 CrossRef CAS PubMed.
- H. D. B. Jenkins, D. Tudela and L. Glasser, Inorg. Chem., 2002, 41, 2364–2367 CrossRef CAS PubMed.
- F. Trouton, Philos. Mag., 1884, 18, 54–57 CrossRef.
- (a) M. J. Kamlet and S. J. Jacobs, J. Chem. Phys., 1968, 48, 23–35 CrossRef CAS PubMed; (b) M. J. Kamlet and J. E. Ablard, J. Chem. Phys., 1968, 48, 36–42 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1028788, 1041566, 1028789 and 1041345. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra00175g |
| This journal is © The Royal Society of Chemistry 2015 |





