DOI:
10.1039/C5RA01682G
(Paper)
RSC Adv., 2015,
5, 28932-28937
Bifurcated hydrogen bonding mediated planar 9,10-anthraquinone dyes: synthesis, structure and properties†
Received
28th January 2015
, Accepted 19th March 2015
First published on 19th March 2015
Abstract
By acylation of mono- and diamino-9,10-anthraquinones with o-alkoxylbenzene carbonyl chloride or o-alkoxylnaphthalene carbonyl chloride, a series of planar 9,10-anthraquinone dyes were designed and synthesized. Because of the formation of bifurcated hydrogen bonds, these dyes adopted planar conformations, which were exemplified by the crystal structure of one dye. The UV-vis absorption spectra and FL (fluorescence) spectra of the dyes were also recorded. The extent of acylation and the positions of the amino and/or amide groups substantially affected the dyes' properties.
Introduction
Anthraquinone dyes are the second largest class of textile pigments besides carcinogenic azo dyes.1–3 They are widely used for the coloration of cotton and cellulose fibers as well as for synthetic materials such as polyamides.4–7 Among these, anthraquinones with amino and hydroxyl substituents have gained special attention due to their photochemical and radiation chemical activities,8,9 and also due to their wide pharmacological and biochemical applications.10 Amino groups present on the 1-, 4-, 5-, 8-positions of 9,10-anthraquinone dyes can form S(6) type11–13 hydrogen bonds with the quinone oxygen atoms14–16 (Fig. 1, left), which are good hydrogen bond acceptors. But amino NH2 is not a good hydrogen bond donor and the hydrogen bond is not strong enough to control the conformation of the whole molecule. We envisioned that if these amino groups are acylated to become amide groups, NHs of the amide groups are good hydrogen bond donors and the hydrogen bonding interaction will become stronger.17–19 Further more, if another hydrogen bond acceptor is properly located as illustrated in Fig. 1, another S(6) type hydrogen bond will form between NH and the ether oxygen atom. Thus an amide NH contacts with two hydrogen bond donors to form a bifurcated hydrogen bond20,21 (Fig. 1, right). The high strength of this bifurcated hydrogen bond will control the whole molecule to be planar. The anthraquinone motif will form a large conjugated system with the terminal benzene or naphthalene motifs by virtue of successive S(6) type hydrogen bonds. The successful control of conformation will lead to special absorption and emission properties.
 |
| | Fig. 1 From amino-anthraquinone to amide-anthraquinone, with bifurcated hydrogen bonding highlighted. | |
In this article, we describe the synthesis of ten planar 9,10-anthraquinone dyes with bifurcated hydrogen bonding controlling their conformation. A 2-substituted dye that does not incorporate a bifurcated hydrogen bond was also synthesized as control compound. X-Ray single crystal structure analysis of one of the dyes confirmed its planar conformation. Absorption and fluorescence emission spectra for 15 dyes (including four commercially available dyes) were recorded.
Experiment section
The syntheses of the target dyes were straight forward as outlined in Schemes 1–3. The corresponding carboxylic acids with an alkoxyl group presenting at the ortho position were first converted to their acyl chlorides upon refluxing in thionyl chloride, and then the acyl chlorides reacted directly with amino-derived anthraquinones in the presentence of triethylamine as base to give the target compounds. Because of strong electron withdrawing ability of the quinone oxygen atoms, the electron density of the amino groups is low and the reactivity is greatly reduced. Even over two equivalent acyl chlorides were used for the above coupling reactions, we separated mono-acylated products with diaminoanthraquinones as reagents. As control, dye 15, where a bifurcated hydrogen bond is not expected, was also synthesized. All the target compounds were thoroughly characterized by 1H NMR, 13C NMR, HRMS, UV-vis and FL spectroscopy.
 |
| | Scheme 1 Synthetic routes for dyes 4–7. | |
 |
| | Scheme 2 Synthetic routes for dyes 8–11. | |
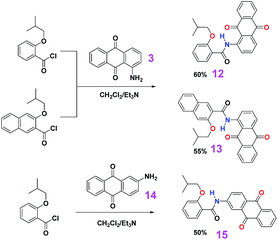 |
| | Scheme 3 Synthetic routes for dyes 12, 13 and 15. | |
General procedure for the preparation of acyl chloride
A solution of appropriate carboxylic acid (usually 10 mmol) in 10 mL thionyl chloride was heated under reflux with a calcium chloride drying tube equipped for 8 hours. Then the solvent was removed under reduced pressure. The crude acyl chloride was used directly for the next step without further purification and characterization.
General procedure for acylation
A solution of the above prepared acyl chloride (usually 10 mmol) in CH2Cl2 (30 mL) was added into a suspension of 1,5-diaminoanthraquinone (1.19 g, 5 mmol), or 1,4-diaminoanthraquinone (1.19 g, 5 mmol), or 1-aminoanthraquinone (2.23 g, 10 mmol), or 2-aminoanthraquinone (2.23 g, 10 mmol), and triethylamine (2.1 mL, 15 mmol) in CH2Cl2 (40 mL) dropwise over a period of 30 minutes with an ice-water bath equipped. After addition, the ice-water bath was removed, and the reaction mixture was heated to reflux. After 9 hours, more CH2Cl2 (100 mL) was added. The solution was washed with 2 N diluted HCl (30 mL), saturated Na2CO3 aqueous solution (30 mL) and saturated brine (30 mL) successively. The organic layer was dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure. The crude product was subjected to column chromatography. Usually for the diaminoanthraquinones, di- and mono-acylated products were both separated.
Characterization data for dyes
All solvents for reactions and column chromatography were used directly as received. Melting points were uncorrected. 1H and 13C NMR spectra were recorded on a Bruker AV 400 MHz or 300 MHz instruments. Chemical shifts were expressed in parts per million (δ) using residual solvent protons as internal standards. Chloroform (δ = 7.26 ppm) was used as an internal standard for chloroform-d. Alcohol free chloroform was used as solvent for spectroscopic measurements, which was thoroughly washed with distilled water and freshly distilled from P2O5. UV-vis data were recorded on UV-2501 PC SHIMADZU and the FL measurements in solution on a Perkin-Elmer Precisely LS45.
Dye 4. Yield: 64%; color: yellow. Mp: 244–245 °C. 1H NMR (300 MHz, CDCl3, TMS, 298 K, ppm): δ 12.98 (s, 2H, NH), 9.32 (d, J = 7.9 Hz, 2H, ArH), 8.09–8.01 (m, 4H, ArH), 7.80 (t, J = 8.1 Hz, 2H, ArH), 7.50 (td, J = 7.5 Hz, J = 1.5 Hz, 2H, ArH), 7.13–7.04 (m, 4H, ArH), 4.10 (d, J = 6.9 Hz, 4H, OCH2), 2.36–2.20 (m, 2H, CH), 0.97 (d, J = 6.7 Hz, 12H, CH3). 13C NMR (75 MHz, CDCl3, TMS, 298 K, ppm): δ 185.6, 166.1, 157.0, 141.5, 135.3, 135.0, 133.2, 132.0, 127.4, 123.7, 122.4, 120.8, 118.4, 112.7, 75.5, 27.7, 19.3. FT-IR (KBr, cm−1): 3203, 3158, 3115, 2967, 2957, 2924, 2905, 2867, 1665, 1651, 1595, 1576, 1502, 1479, 1469, 1450, 1405, 1339, 1310, 1269, 1027, 753, 706. MS: m/z (EI) 590 (M+), 414, 238, 177, 121 (100). Elemental analysis calcd (%) for C36H34N2O6·1/2H2O: C 72.10, H 5.88, N 4.67; found: C 72.35, H 5.88, N 4.71.
Dye 5. Yield: 30%; color: orange. Mp: 172.3–172.7 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.06 (s, 1H, NH), 9.26 (d, J = 8.5 Hz, 1H, ArH), 8.10 (d, J = 7.5 Hz, 1H, ArH), 8.03 (d, J = 7.6 Hz, 1H, ArH), 7.80 (t, J = 8.0 Hz, 1H, ArH), 7.58 (d, J = 7.3 Hz, 1H, ArH), 7.48 (q, J = 7.3 Hz, 2H, ArH), 7.08 (t, J = 7.8 Hz, 2H, ArH), 6.97 (d, J = 8.1 Hz, 1H, ArH), 4.06 (d, J = 6.8 Hz, 2H, OCH2), 2.32–2.20 (m, 1H, CH), 0.95 (d, J = 6.3 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 186.3, 184.7, 166.1, 157.0, 150.7, 141.4, 135.5, 135.3, 135.1, 134.8, 133.0, 131.9, 126.4, 124.1, 122.7, 122.1, 120.8, 118.8, 117.1, 113.2, 112.7, 75.4, 27.8, 19.3. MS: m/z (EI) 414 (M+), 238 (100), 177, 121.
Dye 6. Yield: 40%; color: orange. Mp: 177.3–177.9 °C. 1H NMR (300 MHz, CDCl3, TMS, 298 K, ppm): δ 13.10 (s, 2H, NH), 9.28 (s, 2H, ArH), 8.23 (q, J = 3.3 Hz, J = 2.5 Hz, 2H, ArH), 8.07 (dd, J = 8.7 Hz, J = 1.8 Hz, 2H, ArH), 7.78 (q, J = 3.3 Hz, J = 2.5 Hz, 2H, ArH), 7.49 (td, J = 8.7 Hz, J = 1.8 Hz, 2H, ArH), 7.08 (t + d, J = 8.0 Hz, 4H, ArH), 4.08 (d, J = 6.9 Hz, 4H, CH2), 2.37–2.22 (m, 2H, CH), 0.98 (d, J = 6.7 Hz, 12H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 185.9, 165.9, 157.0, 137.9, 134.1, 133.6, 133.2, 132.1, 130.1, 126.8, 123.6, 120.9, 118.8, 112.6, 75.5, 27.8, 19.3. HRMS (ESI+) calcd for [C36H34N2O6 + H]+ 591.2495, found: 591.2492; calcd for [C36H34N2O6 + Na]+ 613.2315, found: 613.2310.
Dye 7. Yield: 45%; color: red. Mp: 142–143 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.09 (s, 1H, NH), 9.02 (d, J = 9.6 Hz, 1H, ArH), 8.31 (d, J = 7.5 Hz, 1H, ArH), 8.23 (d, J = 7.4 Hz, 1H, ArH), 8.05 (d, J = 7.6 Hz, 1H, ArH), 7.82–7.70 (m, 2H, ArH), 7.48 (t, J = 7.9 Hz, 1H, ArH), 7.08 (t, J = 8.3 Hz, 3H, ArH), 4.06 (d, J = 6.8 Hz, 2H, CH2), 2.30–2.22 (m, 1H, CH), 0.97 (d, J = 6.2 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 185.8, 184.6, 165.8, 157.0, 148.2, 134.2, 134.1, 133.8, 133.7, 133.0, 132.9, 131.8, 131.0, 126.7, 126.5, 125.6, 124.0, 120.8, 118.3, 112.7, 111.5, 75.5, 27.8, 19.3. HRMS (ESI+) calcd for [C25H22N2O4 + H]+ 415.1658, found: 415.1656; calcd for [C25H22N2O4 + Na]+ 437.1477, found: 437.1475; calcd for [2 × C25H22N2O4 + Na]+ 851.3057, found: 851.3055.
Dye 8. Yield: 50%; color: yellow. Mp: >300 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.08 (s, 2H, NH), 9.38 (d, J = 8.4 Hz, 2H, ArH), 8.57 (s, 2H, ArH), 8.07 (d, J = 7.3 Hz, 2H, ArH), 7.91 (d, J = 7.9 Hz, 2H, ArH), 7.85 (t, J = 8.0 Hz, 2H, ArH), 7.79 (d, J = 8.0 Hz, 2H, ArH), 7.55 (t, J = 7.0 Hz, 2H, ArH), 7.41 (t, J = 7.1 Hz, 2H, ArH), 7.34 (s, 2H, ArH), 4.19 (d, J = 6.5 Hz, 4H, OCH2), 2.41–2.29 (m, 2H, CH), 1.02 (d, J = 6.3 Hz, 12H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 185.7, 166.1, 154.0, 141.4, 136.1, 135.5, 135.0, 133.2, 129.0, 128.4, 128.0, 127.4, 126.4, 125.3, 124.4, 122.6, 118.3, 107.4, 75.4, 27.7, 19.4. HRMS (ESI+) calcd for [C44H38N2O6 + Na]+ 713.2628, found: 713.2629.
Dye 9. Yield: 30%; color: orange. Mp: 207.4–208.0 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.13 (s, 1H, NH), 9.32 (d, J = 8.2 Hz, 1H, ArH), 8.53 (s, 1H, ArH), 8.12 (d, J = 7.3 Hz, 1H, ArH), 7.90 (d, J = 7.8 Hz, 1H, ArH), 7.83 (t, J = 8.0 Hz, 1H, ArH), 7.77 (d, J = 7.9 Hz, 1H, ArH), 7.60–7.50 (m, 2H, ArH), 7.47 (t, J = 7.4 Hz, 1H, ArH), 7.40 (t, J = 7.1 Hz, 1H, ArH), 7.31 (s, 1H, ArH), 6.98 (d, J = 8.0 Hz, 1H, ArH), 4.13 (d, J = 6.8 Hz, 2H, CH2), 2.40–2.25 (m, 1H, CH), 0.99 (d, J = 6.5 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 186.4, 184.7, 166.1, 154.0, 150.8, 141.3, 136.0, 135.5, 135.3, 135.2, 134.9, 132.9, 129.0, 128.2, 128.0, 126.3, 125.7, 124.4, 122.8, 122.3, 118.7, 117.2, 113.1, 107.4, 75.3, 27.7, 19.4. HRMS (ESI+) calcd for [C29H24N2O4 + Na]+ 487.1634, found: 487.1632; calcd for [2 × C29H24N2O4 + Na]+ 951.3370, found: 951.3368.
Dye 10. Yield: 40%; color: orange. Mp: 178.3–179.3 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.23 (s, 2H, NH), 9.41 (s, 2H, ArH), 8.62 (s, 2H, ArH), 8.26 (t, J = 4.4 Hz, 2H, ArH), 7.93 (d, J = 8.2 Hz, 2H, ArH), 7.81 (d, J = 8.0 Hz, 4H, ArH), 7.57 (t, J = 7.6 Hz, 2H, ArH), 7.43 (t, J = 7.4 Hz, 2H, ArH), 7.35 (s, 2H, ArH), 4.20 (d, J = 6.8 Hz, 4H, OCH2), 2.44–2.33 (m, 2H, CH), 1.05 (d, J = 6.5 Hz, 12H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 186.0, 165.9, 154.0, 138.0, 136.0, 134.2, 133.6, 133.3, 130.1, 129.1, 128.3, 128.1, 126.9, 126.3, 125.3, 124.4, 118.7, 107.4, 75.4, 27.7, 19.4. HRMS (ESI+) calcd for [C44H38N2O6 + H]+ 691.2808, found: 691.2805; calcd for [C44H38N2O6 + Na]+ 713.2628, found: 713.2625.
Dye 11. Yield: 45%; color: red. Mp: 207.4–208.0 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.17 (s, 1H, NH), 9.09 (d, J = 9.4 Hz, 1H, ArH), 8.54 (s, 1H, ArH), 8.31 (d, J = 7.5 Hz, 1H, ArH), 8.23 (d, J = 7.5 Hz, 1H, ArH), 7.89 (d, J = 8.0 Hz, 1H, ArH), 7.76 (m, 3H, ArH), 7.53 (t, J = 7.4 Hz, 1H, ArH), 7.39 (t, J = 7.3 Hz, 1H, ArH), 7.30 (s, 1H, ArH), 7.12 (d, J = 9.4 Hz, 1H, ArH), 4.14 (d, J = 7.0 Hz, 2H, CH2), 2.42–2.28 (m, 1H, CH), 1.00 (d, J = 6.6 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 186.0, 184.6, 165.8, 154.1, 148.2, 135.9, 134.12, 134.09, 133.8, 133.7, 133.1, 132.9, 130.9, 129.0, 128.2, 128.0, 126.7, 126.5, 126.3, 125.8, 125.6, 124.3, 118.2, 111.5, 107.4, 75.4, 27.7, 19.4. HRMS (ESI+) calcd for [C29H24N2O4 + H]+ 465.1814, found: 465.1813; calcd for [C29H24N2O4 + Na]+ 487.1634, found: 487.1632; calcd for [2 × C29H24N2O4 + Na]+ 951.3370, found: 951.3370.
Dye 12. Yield: 60%; color: yellow. Mp: 190.8–191.3 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.02 (s, 1H, NH), 9.33 (d, J = 8.5 Hz, 1H, ArH), 8.32–8.24 (m, 2H, ArH), 8.12 (d, J = 7.8 Hz, 1H, ArH), 8.05 (d, J = 8.0 Hz, 1H, ArH), 7.82 (t + d, J = 7.7 Hz, 3H, ArH), 7.50 (t, J = 8.2 Hz, 1H, ArH), 7.09 (t, J = 6.9 Hz, 2H, ArH), 4.09 (d, J = 6.5 Hz, 2H, CH2), 2.32–2.22 (m, 1H, CH), 0.97 (d, J = 6.8 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 186.0, 183.0, 166.1, 157.0, 141.7, 135.3, 134.3, 134.2, 134.17, 134.10, 133.2, 132.8, 132.0, 127.7, 127.2, 127.0, 123.7, 122.7, 120.9, 118.9, 112.7, 75.4, 27.8, 19.3. HRMS (ESI+) calcd for [C25H21NO4 + H]+ 400.1549, found: 400.1547; calcd for [C25H21NO4 + Na]+ 422.1368, found: 422.1364; calcd for [2 × C25H21NO4 + Na]+ 821.2839, found: 821.2840.
Dye 13. Yield: 55%; color: yellow. Mp: 179.8–180.1 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 13.12 (s, 1H, NH), 9.41 (d, J = 8.5 Hz, 1H, ArH), 8.57 (s, 1H, ArH), 8.33 (t, J = 4.4 Hz, 1H, ArH), 8.29 (t, J = 4.3 Hz, 1H, ArH), 8.17 (d, J = 7.5 Hz, 1H, ArH), 7.93 (d, J = 8.2 Hz, 1H, ArH), 7.88 (t, J = 8.3 Hz, 1H, ArH), 7.85–7.78 (m, 3H, ArH), 7.57 (t, J = 7.7 Hz, 1H, ArH), 7.43 (t, J = 7.5 Hz, 1H, ArH), 7.35 (s, 1H, ArH), 4.19 (d, J = 7.0 Hz, 2H, CH2), 2.41–2.29 (m, 1H, CH), 1.03 (d, J = 6.5 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 186.1, 183.0, 166.0, 153.9, 141.7, 136.0, 135.4, 134.3, 134.2, 134.14, 134.10, 133.1, 132.8, 129.0, 128.3, 128.0, 127.5, 127.2, 127.0, 126.3, 125.4, 124.4, 122.8, 118.8, 107.4, 75.3, 27.7, 19.4. HRMS (ESI+) calcd for [C29H23NO4 + H]+ 450.1705, found: 450.1704; calcd for [C29H23NO4 + Na]+ 472.1525, found: 472.1523; calcd for [2 × C29H23NO4 + Na]+ 921.3152, found: 921.3152.
Dye 15. Yield: 50%; color: yellow. Mp: 188.1–188.5 °C. 1H NMR (400 MHz, CDCl3, TMS, 298 K, ppm): δ 10.52 (s, 1H, NH), 8.50 (d, J = 7.6 Hz, 1H, ArH), 8.37–8.29 (m, 4H, ArH), 8.24 (s, 1H, ArH), 7.80 (t, J = 3.8 Hz, 2H, ArH), 7.54 (t, J = 7.0 Hz, 1H, ArH), 7.16 (t, J = 7.7 Hz, 1H, ArH), 7.06 (d, J = 8.2 Hz, 1H, ArH), 4.05 (d, J = 6.5 Hz, 2H, CH2), 2.45–2.35 (m, 1H, CH), 1.22 (d, J = 6.7 Hz, 6H, CH3). 13C NMR (100 MHz, CDCl3, TMS, 298 K, ppm): δ 182.9, 182.1, 163.8, 157.0, 144.1, 134.7, 134.2, 134.0, 133.8, 133.7, 133.6, 132.7, 129.4, 129.0, 127.2, 127.1, 124.8, 121.7, 120.8, 117.0, 112.5, 76.0, 28.6, 19.5. MS (ESI+) calcd for [2 × C25H21NO4 + Na]+ 821.28, found: 821.3.
Results and discussion
1H NMR analysis
1H NMR study (Fig. 2, see ESI Fig. S1† for 1H NMR spectra of dyes 8, 9, 10, 11 and 13) in chloroform first confirmed the formation of bifurcated hydrogen bonds. Very sharp signals for amide NHs, irrespective of mono-acylated or di-acylated dyes, all appeared at a very downfield region, δ > 13 ppm. Furthermore little changes were observed for the 1H NMR spectra in a wide range of concentrations (from 50 mM to 0.5 mM). While the signal for NH in compound 15, where bifurcated hydrogen bonding is not available, appeared at 10.52 ppm. The very downfield chemical shift of NH must result from strong bifurcated intramolecular hydrogen bonding interactions.
 |
| | Fig. 2 Stacked partial 1H NMR spectra of dyes 4, 5, 6, 7, 12 and 15, from bottom up, CDCl3, 400 MHz, 298 K. | |
X-ray single crystal analysis
Direct evidence for the bifurcated hydrogen bonding came from X-ray single crystal analysis of dye 4 (Fig. 3). By slow evaporation of a solution of compound 4 in dichloromethane and methanol, we obtained a needle-like single crystal suitable for X-ray analysis.22 As expected, by incorporating two iso-butyloxy groups into the molecule, four highly favorable S(6) type hydrogen-bonded rings formed. Distances between O (iso-butyloxy) and H (amide), O (anthraquinone carbonyl oxygen) and H (amide), O (iso-butyloxy) and O (anthraquinone carbonyl oxygen) are 2.009 Å, 2.001 Å, 2.802 Å respectively, which are much shorter than the sums of van der Waals radii of O and H (2.7 Å, 26% shorter), O and O (3.04 Å, 7.8% shorter). The angles of O (isobutyloxy oxygen)⋯H–N and the O (anthraquinone carbonyl oxygen)⋯H–N are 135.53° and 132.94° respectively. Because of these strong bifurcated hydrogen bonds, the molecule resides its framework almost at a plane. The dihedral angle between ring A and ring B is just 14.3°. Ring A and ring C are parallel to each other. This planar conformation facilitated its further π–π interaction with other molecules. As illustrated in Fig. 4 typic off-set π–π stacking mode23 is observed. An interlayer distance (vertical displacement) is 3.43 Å, which is substantially smaller than the sum of the van der Waals radii of two sp2 carbon atoms and is comparable to the interlayer distance in graphite (3.4 Å). The horizontal displacement is 3.7 Å. Overall, one third of the anthraquinone ring overlaps. This arrangement can bring about the maximum stacking energy.24
 |
| | Fig. 3 Crystal structure (ball and stick model) of dye 4. All hydrogen atoms except NHs were omitted for clarity. Due to the strong S(6) type hydrogen-bonds, the molecule was predefined to be planar and formed a large conjugated system. | |
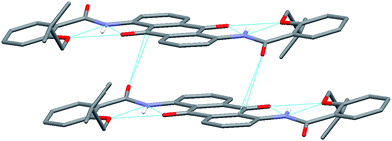 |
| | Fig. 4 Illustration of π–π stacking mode in solid state (capped sticks model). For clarity, all hydrogen atoms except NHs were omitted. | |
UV-vis and FL studies
Upon acylation the dyes displayed different colours. The extent of acylation and the positions of amino and/or amide groups are the two main factors determining the colours. Pictures for each dye in dilute chloroform solution were summarized in Fig. 5. The colours range from red to purple. UV-vis absorption and fluorescence emission spectra for each dye were summarized in Fig. 6–8 and Table 1.
 |
| | Fig. 5 Photos for dyes (from left to right) 1–15, 5 × 10−5 M in chloroform solution. | |
Table 1 Absorption and emission maxima for dyes
| |
1 |
4 |
5 |
8 |
9 |
2 |
6 |
7 |
| Only absorption maxima in the visible region. λex = 430 nm. |
| Absorption maximaa (nm) |
476 |
437 |
466 |
438 |
470 |
544 |
479 |
523 |
| 581 |
| Emission maximab (nm) |
538 |
514 |
548 |
511 |
548 |
604 |
569 |
596 |
| ε (104 L mol−1 cm−1) |
1.41 |
1.36 |
1.17 |
1.61 |
1.36 |
0.94 |
0.88 |
0.83 |
| |
10 |
11 |
3 |
12 |
13 |
14 |
15 |
| Absorption maximaa (nm) |
480 |
523 |
463 |
416 |
416 |
409 |
378 |
| Emission maximab (nm) |
568 |
596 |
541 |
512 |
509 |
523 |
521 |
| ε (104 L mol−1 cm−1) |
0.82 |
1.32 |
0.72 |
0.63 |
0.82 |
0.18 |
0.47 |
For dyes with 1,5-substituted anthraquinone cores (1, 4, 5, 8 and 9, Fig. 6), the acylation of amino groups leads to hypsochromic shift of absorption maxima in the visible light region. For un-acylated dye 1, the absorption maximum is at 476 nm; for mono-acylated dyes 5 and 9, 466 nm and 470 nm are observed respectively; for di-acylated dyes 4 and 8, 437 nm and 438 nm are observed respectively. The terminal groups, benzene or naphthalene, do not affect the absorption spectra substantially. But the terminal groups affect the fluorescence emission intensity substantially. Un-acylated dye 1 shows an emission band at 538 nm. For mono-acylated 5 and 9, the emission maxima are both at 548 nm with almost the same intensity. While for di-acylated 4 and 8, the emission maxima are at 514 nm and 511 nm respectively, and the intensity for the former is larger than the later.
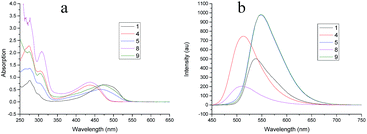 |
| | Fig. 6 (a) UV-vis spectra for dyes 1, 4, 5, 8 and 9, 5 × 10−5 M in chloroform; (b) fluorescence emission spectra for dyes 1, 4, 5, 8 and 9, 1 × 10−5 M in chloroform, λex = 430 nm. | |
For dyes with 1,4-substituted anthraquinone cores (2, 6, 7, 10 and 11, Fig. 7), similar trend was observed for absorption spectra: the absorption maxima in the visible light region showed hypsochromic shift upon acylation, from 544 nm and 581 nm (dye 2, un-acylated) to 523 nm (7 and 11, mono-acylated) to 480 nm (6 and 10, di-acylated). But conditions are quite different for fluorescence behavior. The un-acylated 2 is almost non-emissive when excited with 430 nm. The mono-acylated products 7 and 11 show moderate emission. Greatly enhanced emission is observed for the di-acylated products 6 and 10.
 |
| | Fig. 7 (a) UV-vis spectra for dyes 2, 6, 7, 10 and 11, 5 × 10−5 M in chloroform; (b) fluorescence emission spectra for dyes 2, 6, 7, 10 and 11, 1 × 10−5 M in chloroform, λex = 430 nm. | |
For the third type of dyes with 1-substituted anthraquinone cores (3, 12 and 13, Fig. 8), the absorption follows the above mentioned trend: acylation leads to hypsochromic shift. Dye 14 and 15 are almost UV-vis transparent: very weak absorption are observed. But the fluorescence behavior runs out of the above framework: compared with 1-amino-9,10-anthraquinone 3, the emission is substantially quenched when acylation with o-alkoxynaphthalene carbonyl chloride (dye 13) and a little enhanced with o-alkoxybenzene carbonyl chloride (dye 12). Dye 14 with an amino group at position 2 shows very strong emission intensity, but acylation of the amino group leads to almost completely quenching of the fluorescence (dye 15).
 |
| | Fig. 8 (a) UV-vis spectra for dyes 3, 12, 13, 14 and 15, 5 × 10−5 M in chloroform; (b) fluorescence emission spectra for dyes 3, 12, 13, 14 and 15, 1 × 10−5 M in chloroform, λex = 430 nm. | |
Conclusions
In summary, we introduced bifurcated hydrogen bonding strategy to mediate planar conformation of 9,10-anthraquinone dyes by acylation of amino groups at 1-, 4-, and 5-positions. 1H NMR and X-ray single crystal analysis confirmed the success of our strategy. After acylation of the amino groups the whole dye molecule adopts planar conformation and becomes a large conjugated system, which substantially reduces the energy of the π orbit. Thus the maximum absorption wavelength, which corresponds to π–π* transition, shows hypsochromic shift. Furthermore, the extent of acylation and the positions of functional groups also substantially affect the spectroscopic property of dyes. This strategy of bifurcated hydrogen bonding will bring about new idea and platform for the design and synthesis of new anthraquinone dyes.
Acknowledgements
This work was supported by the Program for Zhejiang Leading Team of Science and Technology Innovation (no. 2010R50038-18), Zhejiang Natural Science Foundation (Grant LY12B02021) and 521 talent program of Zhejiang Sci-Tech University.
Notes and references
- J. R. Aspland, Textile Dyeing and Coloration, American Association of Chemists and Colorists: Research Triangle Park, NC, USA, 1997, p. 251 Search PubMed.
- Dyes, in Encyclopedia of Chemical Technology, ed. R. E. Kirk and D. F. Othmer, Wiley, Hoboken, NJ, 2005, vol. 9, pp. 300–347 Search PubMed.
- A. K. Mishra, J. Jacob and K. Müllen, Dyes Pigm., 2007, 75, 1–10 CrossRef CAS.
- J. S. Manson and D. R. A. Ridyard, Anthraquinone Dyes, US Pat., 3935248, Jan 27, 1976 Search PubMed.
- E. Marechal, Prog. Org. Coat., 1982, 10, 251–287 CrossRef CAS.
- J. Miley, Pure Appl. Chem., 1996, 68, 1423–1428 CrossRef CAS.
- C. Dollendorf, S. K. Kreth, S. W. Choi and H. Ritter, Beilstein J. Org. Chem., 2013, 9, 453–459 CrossRef CAS PubMed.
- T. Yatsuhashi and H. Inoue, J. Phys. Chem. A, 1997, 101, 8166–8173 CrossRef CAS.
- J. P. Rasimas and G. J. Blanchard, J. Phys. Chem., 1995, 99, 11333–11338 CrossRef CAS.
- Y. Duan, J. Yu, S. Liu and M. Ji, Med. Chem., 2009, 5, 577–582 CrossRef CAS.
- M. C. Etter, Acc. Chem. Res., 1990, 23, 120–126 CrossRef CAS.
- M. C. Etter, J. C. MacDonald and J. Bernstein, Acta Crystallogr., Sect. B: Struct. Sci., 1990, 46, 256–262 CrossRef.
- J. Bernstein, R. E. Davis, L. Shimoni and N.-L. Chang, Angew. Chem., Int. Ed. Engl., 1995, 34, 1555–1573 CrossRef CAS.
- P. Dahiya, D. K. Maity, S. K. Nayak, T. Mukherjee and H. Pal, J. Photochem. Photobiol., A, 2007, 186, 218–228 CrossRef CAS.
- P. Dahiya, M. Kumbhakar, T. Mukherjee and H. Pal, J. Mol. Struct., 2006, 798, 40–48 CrossRef CAS.
- P. Dahiya, M. Kumbhakar, D. K. Maity, T. Mukherjee, A. B. R. Tripathi, N. Chattopadhyay and H. Pal, J. Photochem. Photobiol., A, 2006, 181, 338–347 CrossRef CAS.
- F. H. Beijer, R. P. Sijbesma, J. A. J. M. Vekemans, E. W. Meijer, H. Kooijman and A. L. Spek, J. Org. Chem., 1996, 61, 6371–6380 CrossRef CAS PubMed.
- H.-Y. Hu, J.-F. Xiang, J. Cao and C.-F. Chen, Org. Lett., 2008, 10, 5035–5038 CrossRef CAS PubMed.
- H.-Y. Hu, J.-F. Xiang and C.-F. Chen, Org. Biomol. Chem., 2009, 7, 2534–2539 CAS.
- Z.-Q. Wu, X.-K. Jiang, S.-Z. Zhu and Z.-T. Li, Org. Lett., 2004, 6, 229–232 CrossRef CAS PubMed.
- R. D. Parra, H. Q. Zeng, J. Zhu, C. Zheng, X. C. Zeng and B. Gong, Chem.–Eur. J., 2001, 7, 4352–4357 CrossRef CAS.
- Crystal data: C36H34N2O6, Mw = 590.65, crystal size: 0.79 × 0.15 × 0.13 mm3, crystal system: monoclinic, space group P2(1)/n, a = 5.1550(10), b = 23.851(5), c =11.944(2) Å, β = 93.18(3)°, U = 1466.3(5) Å3, Z = 2, Dc = 1.338 Mg m−3, T = 293(2) K, μ (Mo-Kα) = 0.71073 mm−1, 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881 reflections measured, 3297 unique (Rint = 0.0623), R1 = 0.0506, wR2 = 0.1091 (ESI†).
881 reflections measured, 3297 unique (Rint = 0.0623), R1 = 0.0506, wR2 = 0.1091 (ESI†). - C. A. Hunter and J. K. M. Sanders, J. Am. Chem. Soc., 1990, 112, 5525–5534 CrossRef CAS.
- S. Toyota, M. Goichi and M. Kotani, Angew. Chem., Int. Ed., 2004, 43, 2248–2251 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra for all dyes and CIF for the single crystal of dye 4. CCDC 250831. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra01682g |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881 reflections measured, 3297 unique (Rint = 0.0623), R1 = 0.0506, wR2 = 0.1091 (ESI†).
881 reflections measured, 3297 unique (Rint = 0.0623), R1 = 0.0506, wR2 = 0.1091 (ESI†).




