Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Porous membranes designed from bi-phasic polymeric blends containing silver decorated reduced graphene oxide synthesized via a facile one-pot approach
Prasanna
Kumar S Mural
a,
Maya
Sharma
a,
Abhinaya
Shukla
b,
Sambhu
Bhadra
b,
Babu
Padmanabhan
b,
Giridhar
Madras
c and
Suryasarathi
Bose
 *d
*d
aCenter for Nano Science and Engineering, Indian Institute of Science, Bangalore-560012, India
bPolymer Science Diagnostic Center, Steer Engineering Pvt. Ltd, Bangalore-560058, India
cDepartment of Chemical Engineering, Indian Institute of Science, Bangalore-560012, India
dDepartment of Materials Engineering, Indian Institute of Science, Bangalore-560012, India. E-mail: sbose@materials.iisc.ernet.in
First published on 31st March 2015
Abstract
In this work, porous membranes were designed by selectively etching the PEO phase, by water, from a melt-mixed PE/PEO blend. The pure water flux and the resistance across the membrane were systematically evaluated by employing an indigenously developed cross flow membrane setup. Both the phase morphology and the cross sectional morphology of the membranes was assessed by scanning electron microscopy and an attempt was made to correlate the observed morphology with the membrane performance. In order to design antibacterial membranes for water purification, partially reduced graphene oxide (rGO), silver nanoparticles (Ag) and silver nanoparticles decorated with rGO (rGO–Ag) were synthesized and incorporated directly into the blends during melt mixing. The loss of viability of bacterial cells was determined by the colony counting method using E. coli as a model bacterium. SEM images display that the direct contact with the rGO–Ag nanoparticles disrupts the cell membrane. In addition, the rGO–Ag nanoparticles exhibited a synergistic effect with respect to bacterial cell viability in comparison to both rGO and Ag nanoparticles. The possible mechanism associated with the antibacterial activity in the membranes was discussed. This study opens new avenues in designing antibacterial membranes for water purification.
1 Introduction
The demand for pure water is a necessity in view of the rapidly increasing population, especially in developing nations. Fresh water conservation and purification of the existing sources has become indispensable in order to meet this global water demand. This requires better water management.1 Among the various available water treatment technologies, such as media filtration, distillation or disinfection; separation technology is favored due to lower energy consumption, cost and it has no requirement for chemical additives.1 Among the various membrane separation technologies, pressure driven separation is the most widely used method.2,3Polymeric membranes are often used in the filtration technology due to their competitiveness in performance and economy.4 Polymeric membranes are commercially prepared by various methods like thermally induced phase separation (TIPS), stretching melt-cast polymer films and track etching.5 The polymeric membranes that are commercially available are made up of cellulose acetate, nitrocellulose, and cellulose esters, polysulfone, polyether sulfone, polyacrylonitrile, polyamide, polyimide, polypropylene, polytetrafluoroethylene, polyvinylidene fluoride and polyvinylchloride.5 Among the different polymers that are commercially available, polyolefins are often of commercial interest due to their low, good processability and chemical resistance.6
Recently, Trifkovic et al.7 generated porous structures by selectively etching one of the phases from bi-phasic polymeric blends. This method led to generation of porous structure with desired morphology and microstructure. Thus polymer blending offers myriad opportunities to design porous structures that can further be explored for separation technology. Polymer blends can, in general, be prepared either by solution mixing or by melt blending. The latter technique offers numerous advantage in designing new materials and hence an industrially viable technique.8 It has been realized that blending two polymers often exhibit thermodynamic immiscibility, which leads to coarse morphology due to large interfacial tension between the phases.9 Different morphologies such as droplet/matrix, fiber, lamella, and co-continuous are often obtained by blending and can be tuned by varying the physical properties like interfacial tension, viscosities, volume fractions and the processing parameters.10 Among the different morphologies, the matrix/droplet morphology can be utilized for various applications, especially for separation technology by selectively removing the minor phase.
One of the major concerns in separation technology especially for water purification is that the membranes tend to foul over a period of time due to metabolic activities of the bacteria cells.11–13 Fouling tends to decrease the permeate flux resulting in poor performance of the membrane. In recent years, nanoparticles such as graphene oxide (GO), silver (Ag), copper (Cu), zinc oxide (ZnO), and titanium oxide (TiO2) etc., have shown cytotoxicity to a broad spectrum of microorganisms.14–16 However, studies related to the use of these nanoparticles in polymer matrix for membrane applications are still very scarce. GO sheets have sharp edges, which can physically damage the cell membrane leading to cell lysis.17 Moreover, GO has a tendency to induce oxidative stress on the cell membrane.18 On the other hand, Ag nanoparticles show antimicrobial activity19via release of silver ions (Ag+) and the generation of reactive oxygen species (ROS).13 It is well reported that the critical concentration for Ag nanoparticles that can kill bacterial cells is 0.001 μg L−1.20 However, Ma et al.21 showed that Ag–GO composite powder can show very high bactericidal activity. This was attributed to the decrease in the surface charge that enhances the contact between the bacterial cell and the composite powder.
In this work, a unique strategy was adopted to design PE based porous membranes for water purification. PE/PEO (90/10, wt/wt) blends were melt mixed and the water soluble PEO phase was etched out to develop well defined porous structures. Pure water flux and the resistance across the membrane were evaluated using an indigenously developed cross flow cell. Different nanoparticles such as partially reduced graphene oxide (rGO), silver nanoparticles decorated rGO (rGO–Ag) were synthesized to impart antibacterial property to the membrane and more importantly, impede biofilm formation. The latter often results in clogging the pores and reduces the overall flux over a period of time. The efficacy of antibacterial activity was systematically assessed by using Escherichia coli (E. coli) as model bacterium. Further, the possible mechanisms associated with bacterial cell viability were also discussed.
2 Experimental
Materials
Low density polyethylene (PE with melt flow index of 25 g/10 min), polyethylene oxide (PEO, Mv of 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from Sigma Aldrich. Silver nanoparticles and silver nitrate (AgNO3) were procured from Merck (India). The size of the silver nanoparticles was ca. 20 nm. All other solvents and reagents were of analytical grade and were used as received.
000) was obtained from Sigma Aldrich. Silver nanoparticles and silver nitrate (AgNO3) were procured from Merck (India). The size of the silver nanoparticles was ca. 20 nm. All other solvents and reagents were of analytical grade and were used as received.
Synthesis of graphene oxide (GO)
GO was synthesized by using an improved method as described in our previous work.6 Briefly, graphite flakes were mixed with of concentrated H2SO4 and H3PO4 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) in a water bath with the slow addition of KMnO4 and the mixture was stirred for 24 h at room temperature. Upon completion of reaction, hydrogen peroxide was added to stop the reaction and then solution was filtered and washed successively with DI water, HCl and ethanol. Thus, the obtained solid (GO) was vacuum-dried at room temperature.
1 ratio) in a water bath with the slow addition of KMnO4 and the mixture was stirred for 24 h at room temperature. Upon completion of reaction, hydrogen peroxide was added to stop the reaction and then solution was filtered and washed successively with DI water, HCl and ethanol. Thus, the obtained solid (GO) was vacuum-dried at room temperature.
Synthesis of silver decorated partially reduced graphene oxide (rGO–Ag)
rGO–Ag was obtained by the method described elsewhere.22 Briefly, 1 wt% of GO was added to ethylene glycol and sonicated for 30 min followed by 1 h of stirring. 200 mg of AgNO3 was dissolved in a mixture of ethylene glycol and distilled water (DI). Thus obtained AgNO3 mixture was added to GO–ethylene glycol mixture and the resultant solution was kept under constant stirring for 2 h at 50 °C. Further, NaBH4 (40 mL of 0.1 mol L−1) was added slowly and the mixture was heated to 110 °C and kept for 2 h under constant stirring. After completion of reaction, the mixture was washed and filtered using DI and then vacuum dried at 80 °C. In order to synthesize rGO, all the steps mentioned above were followed except the addition of AgNO3, ethylene glycol and DI mixture.Preparation of blends
Neat blends of PE/PEO (90/10 wt/wt) and with rGO, rGO–Ag nanoparticles were melt mixed using a twin screw extruder (Polylab, Thermo Haake Minilab II) at 150 °C and 60 rpm for 20 min under N2 atmosphere. Homogeneous mixing was ensured by the recirculation channel in the Minilab II. Prior to mixing, all the samples were kept in a vacuum oven overnight to remove any traces of moisture. Blends were also prepared with GO and Ag.Characterization of the nanoparticles
The characteristic dimensions of the synthesized nanoparticles were determined using field emission scanning electron microscopy (SEM) (ULTRA 55, FESEM, Carl Zeiss) with accelerating a voltage of 5 kV and transmission electron microscopy (TEM, Tecnai G2 T20). The presence of functional moieties was confirmed using spectroscopic techniques. Fourier transform infrared (FTIR) spectra were recorded on a Perkin-Elmer Frontier in the range of 400–4000 cm−1. GO and rGO were further characterized using Raman spectroscopy using Horiba LabRAM HR with a 532 nm monochromatic laser. Diffraction patterns were acquired by X-ray diffraction (XRD) technique using a Rigaku Cu Kα source. X-ray Photon Scattering (XPS) was performed by Kratos Analytical instrument using Al monochromatic source.The phase morphologies of the various blends and the cross sectional morphologies of the membranes were studied using cryo fractured surface by FESEM at 5 kV accelerating voltage. Prior to the morphological analysis, PEO was etched out with cold water to enhance the contrast between the phases. The mechanical properties of the blend was analyzed using uniaxial tensile test (as per ASTM D368 type V samples) using Instron Universal Testing Machine at room temperature with a cross head speed of 5 mm min−1. Surface wettability measurements and the contact angle measurements were performed using sessile drop water goniometry.
Membrane performance
The performance of the various membranes was analyzed by estimating the permeate flux (J) using an indigenously developed cross flow setup. Samples were hot pressed into discs and polished from both the sides as the flow properties of both PE and PEO are very different.6 The following procedure was used to measure the pure water flux. The trans-membrane pressure was varied from 2.5 to 12.5 psi with a step change of 2.5 psi. It is important to mention here that prior to the measurements, the pressure was maintained for 30 min to obtain a steady flow and then the permeate flux was recorded. For stability and reproducibility of data at least three samples were tested. The experiments were repeated until three consecutive readings were within 10% of each reading. Permeate flux is defined as, J = Q/A × t. Where Q (L) is the volume of permeated water, A (m2) is area of the substrate and t (s) is the time taken by permeate to fill the volume (Q).Antibacterial performance
The antibacterial activities of the membranes were evaluated using a wild culture of Gram-negative bacteria (Escherichia coli). Firstly, E. coli was sub cultured in Luria Bertani (LB) broth at 37 °C for 3 h (till mid log phase). Thus cultured E. coli was pelletized and washed with phosphate buffered saline (PBS) to remove nutrients from the broth. Pellets were re-suspended in PBS and diluted to reach the required concentration of 109 CFU mL−1. The membranes of known dimensions were incubated in the PBS containing E. coli for 12 h. After 12 h, the E. coli cells were agar plated and the colonies formed after 24 h were assessed. The adhesion of the E. coli was analyzed by E-SEM. The samples were prepared by fixing the bacteria on the surface with 2.5% glutaraldehyde followed by rinsing by distilled water (DI) and dried under vacuum.3 Results and discussion
Synthesis and characterization of GO, rGO and rGO–Ag
GO was synthesized using a modified Hummers method, where graphite was oxidized using KMnO4 in the presence of H2SO4. The oxidation of graphite to GO was confirmed by using FTIR (Fig. 1) where a broad band centered around 3200 cm−1 is evident corresponding to the phenol hydroxyl groups on the basal plane. In addition, the characteristic stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carboxylic) at 1726 cm−1, the C
O (carboxylic) at 1726 cm−1, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C sp2 hybridization at 1626 cm−1 and the epoxide group at 1050 cm−1 further confirms the oxidation of graphite to GO.
C sp2 hybridization at 1626 cm−1 and the epoxide group at 1050 cm−1 further confirms the oxidation of graphite to GO.
Reduction of GO to rGO and Ag on to rGO22 involves the use of ethylene glycol as a chelating and a mild reducing agent along with NaBH4 (a strong reducing agent to enhance the rate of reaction). FTIR spectra of rGO–Ag indicates reduction in the intensities of hydroxyl and the carboxyl stretching which clearly manifests in the in situ reduction of GO in the presence of ethylene glycol and NaBH4 during the synthesis of rGO–Ag. This presence of hydroxyl groups on the basal planes of GO is responsible for the interaction of Ag nanoparticles with the GO sheets.22
The Raman spectra of GO, rGO and rGO–Ag are shown in Fig. 2. From the spectra, it is well evident that the signature peaks of graphitic structures like D band (1333 cm−1) and G band (1598 cm−1) are present. The D band arises due to defects namely edge and disordered carbon. Similarly G band indicates the sp2-bonded carbon i.e. graphitic structures. The ratio of intensities of D band (ID) to G band (IG) is indicative of degree of disorder and number of defects in the graphene sheet. For rGO, a slight increase in ID/IG ratio from 1.12 for GO to 1.15 is observed, which can be attributed to an increase in defects on account of reduction of oxygen sites. The ID/IG ratio increased further to 1.24, thus suggesting more defects in rGO–Ag, consistent with the literature.22 It is envisaged that upon reduction of GO, the defects increases due to removal of the functional group and reduction of the sp2 domains. Hence, an increase in ID/IG ratio is observed during reduction of GO.
XRD (Fig. 3) of GO shows a peak at 10.19° 2θ and on reduction with ethylene glycol and NaBH4, the peak centered around 20–30° can be attributed to the reflection of plane (002) of the hexagonal graphene sheet. This decrease in d-spacing and the broadening suggests re-stacking of graphene sheets23 due to the reduction in oxygen groups. Further, rGO–Ag exhibited diffraction lines that can be indexed to Ag face centered cubic structure. The sharp peak at 38.2° suggests an average particle size of ca. 50 nm.
TEM micrograph (Fig. 4) of GO showed a thin, smooth surface along with minor wrinkles along the edges.16 In case of rGO, a thick stack of sheets was observed that can be attributed to the removal of oxygen functional groups leading to the development of secondary bonding which is consistent with the results from XRD and FTIR. TEM micrograph of rGO–Ag clearly shows the deposition of Ag on the rGO sheets. These are marked by black dots on the surface and was confirmed from the diffraction pattern of the (111) plane of Ag.21
 | ||
| Fig. 4 TEM micrographs of (a) GO (b) rGO (c) rGO–Ag and (d) higher magnification micrograph of rGO–Ag (inset shows the corresponding diffraction patterns). | ||
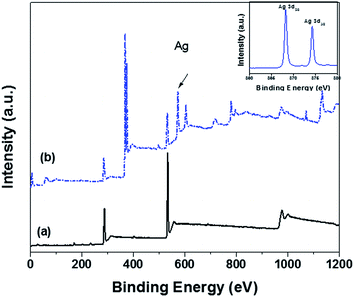
X-ray photon spectra (XPS) of GO and rGO–Ag are shown in Fig. 5. The presence of oxygen is well evident coming from various functional groups like carboxylic at the edge and the phenol hydroxyl and the epoxy groups on the basal plane. Upon reduction of Ag on the surface of GO, the corresponding intensities related to the oxygen species has decreased suggesting loss of oxygen groups during the reduction process. The rGO–Ag particles exhibited a doublet at 368.2 and 374.2 eV, which can be assigned to Ag 3d5/2 and Ad 3d3/2, respectively. Further, the splitting in 3d with a difference of 6.0 eV suggests the formation of metallic silver.24 Interestingly, from the C/O ratios (Table 1), it is clear that the one-pot facile synthesis of rGO–Ag nanoparticles results in more oxygen species on the rGO sheets than the reduction of GO to rGO. From the above results, it is well evident that oxygen species provide nucleating sites for silver and further nucleation is strongly dependent on the degree of oxygen groups present on the surface.25 It is well known phenomena that oxygen moieties are responsible for initial attachment of Ag+ ions by electrostatic interactions. Further, upon reduction of Ag+ ions using NaBH4, the Ag particles grow on these sites. In the reduction of GO to rGO, the oxygen content has reduced in comparison to rGO–Ag nanoparticles which can be attributed to a competing effect. As reduction of Ag+ by NaBH4 is faster, the reduction of Ag is more favored than the reduction of functional groups in the GO sheet. It is further evident from XPS that rGO showed the highest C/O ratio (4.29) than rGO–Ag (1.93) supporting the above hypothesis. Further, presence of Ag on rGO–Ag will inhibit the restacking of GO sheet.26 In case of rGO, the restacking of sheets due to strong van der Waals' forces27 is indispensable and is also supported by TEM where rGO appears larger in size as compared to GO. Interestingly, the rGO–Ag nanoparticles are smaller in size than the rGO sheets possibly due to fragmentation of the sheets during the synthesis of rGO–Ag nanoparticles.
| Particle | Elements | Atomic concentration (%) | Mass concentration (%) | C/O ratio (atomic ratio) |
|---|---|---|---|---|
| GO | C | 61.6 | 55.0 | 1.61 |
| O | 38.4 | 45.0 | ||
| rGO | C | 81.1 | 76.3 | 4.3 |
| O | 18.9 | 23.7 | ||
| rGO–Ag | C | 55.3 | 23.7 | 2.0 |
| O | 28.8 | 16.3 | ||
| Ag | 15.7 | 60.0 |
It is important to note that the Ag nanoparticles did not get oxidized during the preparation of rGO–Ag. To support this hypothesis, XRD was carried out on the rGO–Ag powder which was exposed to air atmosphere for 24 h and on the membrane tokens containing rGO–Ag which was exposed to water for one month (not shown here). Interestingly, we observed identical diffraction patterns for the membrane tokens with 1 wt% rGO–Ag after exposing the membranes to water for a months' time with respect to the as prepared membrane tokens. Similar observations were recorded for the nanoparticles which were exposed to air. This clearly demonstrates the fact that Ag do not oxidize in rGO–Ag nanoparticles.
Phase morphology in PE/PEO blends
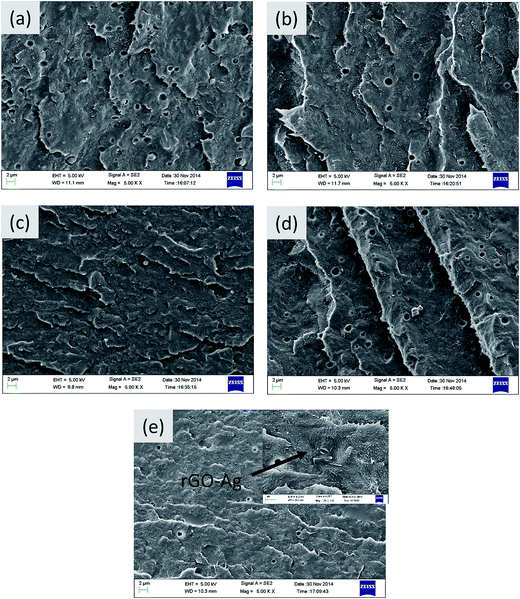
The phase morphology in the blends depends on various parameters such as blend ratio, melt viscosity, processing parameters and also on the presence of nanoparticles etc.28 PE/PEO blends form an immiscible pair and offer different morphologies depending on the viscosity ratio of the constituent polymers.6,7,29,30Fig. 6 displays the SEM micrographs of various blends generated by blending PE (90 wt%) and PEO (10 wt%). The minor phase (i.e. PEO) has been etched out with water to enhance the contrast between the phases and hence, the holes in the micrographs represents the etched out PEO phase. The average pore size in the neat blends is of the order of 1.3 μm.6 Further, with addition of rGO, Ag and rGO–Ag particles a marginal decrease in pore size is observed, which can be due to suppression of coalescence led by the nanoparticles. It is well known that nanoparticles can either increase the viscosity of the matrix phase or can act as a physical barrier between the droplets.31 The nanoparticles selectively localize in the PE phase of the blends, driven by its lower melt viscosity in striking contrast to PEO. This is evident from the SEM micrographs (see inset of Fig. 6d) where rGO–Ag nanoparticles appear as bright features marked by wrinkled edges. This droplet-matrix morphology, thus generated by blending PE and PEO, can be further explored for membrane separation application and is discussed in the next section.Membrane performance
The trans-membrane flux across the membranes was determined using a typical cross flow setup. Prior to the flux measurements, the membranes were prepared by polishing the surface to a depth of 200 nm. This was done to remove the skin layer, which is PE rich as explained in detail in our previous work.6Fig. 7 represents the typical flux measurements as a function of trans-membrane pressure. It is clear from Fig. 7 that incorporation of different nanoparticles has decreased the flux across the membrane and can be attributed to decrease in the pore size. This is also supported by the observed morphological changes, as discussed in the previous section. | ||
| Fig. 7 Typical flux measurement versus trans membrane pressure of 90/10 PE/PEO blends (a) neat (b) with 1 wt% GO (c) with 1 wt% rGO, (d) with 1 wt% Ag and (e) with 1 wt% rGO–Ag. | ||
The resistance offered by the membrane was measured from the reciprocal of slope in Fig. 7. From Table 2 it is clear that with the incorporation of particles the resistance offered by the membranes has increased from 9.02 ± 0.11 psi (L m−2 h−1)−1 for neat blends to 29.43 ± 0.43 psi (L m−2 h−1)−1 with incorporation of rGO–Ag. This clearly suggests a decrease in the pore size resulting from suppression of coalescence. The particles present in the matrix act as a physical barrier thereby prevents droplet coalescence and stabilizes the blend morphology. This reduction in droplet offers higher resistance to the flow which results in an increase in the resistance to flow and decreases the overall flux. Vleminckx et al.32 reported that coarsening of the domain in matrix was suppressed by TRG (thermally reduced graphene) which acts as physical barrier in the blend. Table 2 also depicts the contact angle of the various membranes. The contact angle for blends with GO is ca. 59 ± 2 and can be attributed to various functional groups present on the surface. These surface oxygen groups are substantially reduced in the blends with rGO thereby, making it more hydrophobic.
| Sample | Contact angle (°) | Resistance (psi L−1 m2 s) | Avg. flux@12.5 psi (L m−2 h−1) |
|---|---|---|---|
| Neat | 75 ± 2 | 9.02 ± 0.25 | 4721 ± 134 |
| With 1 wt% GO | 59 ± 2 | 27.34 ± 2.46 | 1633 ± 147 |
| With 1 wt% rGO | 75 ± 3 | 31.12 ± 1.57 | 1386 ± 70 |
| With 1 wt% Ag | 69 ± 3 | 38.18 ± 2.98 | 1243 ± 97 |
| With 1 wt% rGO–Ag | 77 ± 2 | 29.43 ± 1.66 | 1365 ± 77 |
In order to check the compaction in the membrane tokens, we had pressurized the tokens to 10 psi and stabilized it for 1 h and subsequently the pressure was reduced to half and the flux was measured as ca. 637.02 ± 8.18 L m−2 h−1. Prior to these measurements, we initially measured the flux at 5 psi which showed ca. 644.89 ± 39.03 L m−2 h−1. These observations clearly demonstrate the fact that the compaction phenomenon is not pronounced in PE/PEO blends.
Antibacterial activity in the membranes: effect of rGO and rGO–Ag nanoparticles
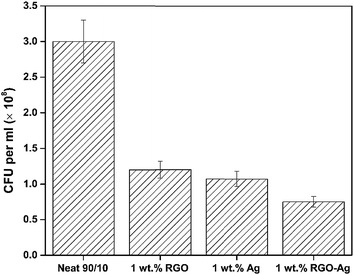
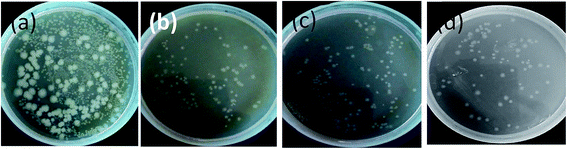
The antibacterial activity of the membranes was evaluated using E. coli.(Gram-negative) as a model bacterium. The antibacterial activity after 24 h in terms of CFU mL−1 of neat, rGO, Ag, and rGO–Ag membranes is shown in Fig. 8. Compared to the membranes derived from neat blends, the membranes with 1 wt% rGO, Ag and rGO–Ag exhibited significantly higher bactericidal effect as observed from the plates where only fewer colonies of E. coli was present.It is envisaged that rGO can lead to cell death due to oxidative stress or physical disruption causing rupture of cell wall.27 In the present study, the mechanism of physical disruption causing rupture of cell is studied in further detail by SEM and will be discussed in the subsequent section. Fig. 9 shows the agar plates after inoculation of E. coli for 12 h. It is clear from these plates that in the case of rGO, the cell count has decreased from 3 × 108 to 1.2 × 108 CFU mL−1. Similarly, in the case of Ag nanoparticles, a significant decrease in the CFU mL−1 was noted. This can be attributed to the disruption of cell membrane function, which can interrupt the electron transport system and further damage the cell proteins and DNA (by binding to sulphur group like thiol group).33 This is also well supported by SEM and will be discussed in the subsequent section. Such a slow rate may be due to slow release of Ag ions from the membranes in the PBS media.33 Muñoz-Bonilla et al.34 recently highlighted that the release of Ag ions is more in hydrophilic polyamide nanocomposites than in hydrophobic polypropylene. In addition, they reported that the slow release of Ag ions will have long term antibacterial behavior. Interestingly, in the membranes with rGO–Ag a synergistic effect was noted. The CFU mL−1 further decreased to 7.5 × 107 cells. Ma et al.21 showed that rGO–Ag showed a decrease in the negative charge in comparison with GO. This reduction in surface charge enhances the contact between the particle and the E. coli cells. We believe that the direct contact of rGO–Ag nanoparticles with E. coli results in synergistic effects in the membrane. This will impede the formation of biofilm formation on the membrane. Sharma et al.35 recently reported a similar phenomenon in PDVF membrane wherein the biofilm was prevented by incorporation of Ag nanoparticles.
Cell viability
The morphological changes in E. coli, when exposed to the membranes, are displayed in Fig. 10. The membranes derived from neat 90/10 PE/PEO blends exhibited a smooth and intact E. coli cells (Fig. 10a) whereas the membranes with the nanoparticles showed disturbed and flattened morphology. From Fig. 10b it is well evident that the sharp edges of rGO might have resulted in disruption of the cell membrane leading to its death. This physical disruption of cell membrane in the presence of rGO can be explained in terms of the nature of the cell. The Gram negative bacteria cells have a thin layer of peptidoglycan of ca. 7–8 nm along with a layer of lipopolysaccharides.36 This peptidoglycan can interact with the rGO sheets. Further, sharp edges of rGO can also disrupt the cell membrane leading to the release of intracellular content (debris). This physical disruption of peptidoglycan leads to lysis of cells.17 | ||
| Fig. 10 Morphological of 90/10 PE/PEO (a) neat (b) with 1 wt% rGO (c) with 1 wt% Ag and (d) with 1 wt% rGO–Ag (arrows indicate cell lysis). | ||
Recently, several studies have comprehensively highlighted the bactericidal properties of silver nanoparticles.37,38Fig. 10c shows the cell morphology in the presence of membranes with Ag nanoparticles. Several mechanisms have been proposed for the loss of cell viability in the presence of Ag. In the present study, from the SEM micrographs, it is evident that due to direct contact with the cells Ag nanoparticles has led to irreversible cell damage finally resulting in cell death. However in the case of rGO–Ag nanoparticles, we believe that the adsorption is much stronger due to decrease in the negative charge, as compared to GO, (refer inset of Fig. 10d). This adsorption prevents bacterial cell nourishment. In addition, direct contact with rGO–Ag might also induce membrane stress resulting in cell lysis. The Gram negative bacteria (such as the one studied here, E. coli) comprise cell wall of thin layer of peptidoglycan (PG) and an outer membrane consisting of lipopolysaccharides. The latter gives negative charge to the cell membrane which is essential for its structural integrity and viability.39 Thus, if the structural integrity of the cell is disturbed then the viability of the cell is lost resulting in cell lysis. The bactericidal effects associated with rGO has been reported with respect to its size, oxidation capacity, physical disruption of membrane or by bridging lipid bilayer to external environment for release of cellular energy.40 GO, due to presence of oxygen group on the surface are insulators and hence can bridging lipid bilayer to external environment for the release of cellular energy is impeded as against rGO, which are conducting. The rGO sheets are capable of interacting with thick cell membrane and the sharp edges disrupts the cell membrane leading to the release of intracellular content and finally resulting in irreversible cell lysis.
Silver nanoparticles disrupts the permeability, respiration, and cell division when it interacts with the cell membrane and the sulfur- and phosphorus-containing compounds.41 It also depends on various factors like particle size, shape and water chemistry.40 Smaller particle size enhances the antibacterial properties due to high specific surface area.41
Interestingly, the rGO–Ag nanoparticles showed synergistic effect in bactericidal properties. This can be attributed to slight positive charge on rGO–Ag as compared to rGO.21 To support our hypothesis, zeta potential for GO, rGO and rGO–Ag was estimated using the zeta potential (−27.4 ± 0.3 mV at 7 pH in distilled water) of Ag as the reference. We observed that GO, rGO and rGO–Ag, showed a zeta potential of −40.28 ± 1.56, −34.44 ± 1.33 and −29.27 ± 1.78 mV respectively, at pH 7. This decrease in zeta potential of rGO–Ag suggests that rGO–Ag exhibit a relatively positive charge and can be attributed to the decrease in functional group and incorporation of positively charged Ag nanoparticles. Hence from zeta potential, we can argue that rGO–Ag possess a positive charge on the surface and this charge is responsible for physiological interaction between the negatively charged lipopolysaccharides and the rGO–Ag nanoparticles leading to cell death.41 In addition, the rGO–Ag nanoparticles exhibited fragmented graphene sheets, as observed in TEM, with sharp edges which can physical disrupt the membrane. As discussed earlier, the oxygen content in rGO–Ag nanoparticles are higher with respect to rGO. This higher content of oxygen species possibly can impart oxidative stress on the cell membrane. It is worth mentioning that we mapped the release of Ag ions from the membranes by AAS (atomic absorption spectroscopy) over a period of 12 h to understand the release kinetics (not shown here). We did not observe any appreciable release of Ag ions that clearly indicates that the bactericidal effects in Ag nanoparticles are via direct contact. This observation can also possibly explain the similar bactericidal effects noted in both Ag and rGO nanoparticles. The factors discussed above explain the synergistic effects in rGO–Ag nanoparticles. An important observation is worth pointing out here. The bactericidal effects rendered by rGO–Ag can possibly impede the biofilm formation. The latter often results in clogging the pores and lead to overall decrease in the flux. Sharma et al.35 recently reported a similar phenomenon in PDVF membrane wherein the biofilm was prevented by incorporation of Ag nanoparticles. It is envisaged that in PE based membranes, the biofilm formation is the major cause for degradation. Hence, by preventing this, the lifetime of the proposed membranes can be enhanced significantly and a detailed investigation in this regard is subjected to future investigation. A cartoon further highlighting the conceptual design of antibacterial membranes is displayed in Fig. 11.
 | ||
| Fig. 11 A cartoon illustrating the key role of rGO–Ag nanoparticles as bactericidal agent in porous PE membranes for water purification. | ||
4 Conclusions
A unique strategy was adopted to design antibacterial membranes in this study. By etching out one of the phases from a binary blend, porous substrates were developed and evaluated for membrane performance. Both the cross section and the phase morphology was corroborated with the observed flux and the resistance offered by the membranes. The effect of rGO, Ag and Ag decorated rGO was evaluated on the bactericidal effect on the membrane. The membranes with rGO–Ag showed synergistic improvement in the bactericidal activity against E. coli. Such membranes can open up new avenues for water purification using a cost effective and easy to process PE based membranes.Acknowledgements
The authors would like to acknowledge the Department of Science and Technology for the financial support, Prof. Jayant Modak for extending his facilities and Materials Engineering Department and CeNSE, IISc for various characterization facility.References
- M. M. Pendergast and E. M. V. Hoek, A review of water treatment membrane nanotechnologies, Energy Environ. Sci., 2011, 4, 1946–1971 CAS.
- R. E. Kesting, The four tiers of structure in integrally skinned phase inversion membranes and their relevance to the various separation regimes, J. Appl. Polym. Sci., 1990, 41, 2739–2752 CrossRef CAS.
- M. Ulbricht, Advanced functional polymer membranes, Polymer, 2006, 47, 2217–2262 CrossRef CAS.
- A. Mehta and A. L. Zydney, Permeability and selectivity analysis for ultrafiltration membranes, J. Membr. Sci., 2005, 249, 245–249 CrossRef CAS.
- G. M. Geise, H.-S. Lee, D. J. Miller, B. D. Freeman, J. E. McGrath and D. R. Paul, Water purification by membranes: the role of polymer science, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 1685–1718 CrossRef CAS.
- P. K. S. Mural, A. Banerjee, M. S. Rana, A. Shukla, B. Padmanabhan, S. Bhadra, G. Madras and S. Bose, Polyolefin based antibacterial membranes derived from PE/PEO blends compatibilized with amine terminated graphene oxide and maleated PE, J. Mater. Chem. A, 2014, 2, 17635–17648 CAS.
- M. Trifkovic, A. Hedegaard, K. Huston, M. Sheikhzadeh and C. W. Macosko, Porous Films via PE/PEO Cocontinuous Blends, Macromolecules, 2012, 45, 6036–6044 CrossRef CAS.
- S. Bose, A. R. Bhattacharyya, A. R. Kulkarni and P. Pötschke, Electrical, rheological and morphological studies in co-continuous blends of polyamide 6 and acrylonitrile–butadiene–styrene with multiwall carbon nanotubes prepared by melt blending, Compos. Sci. Technol., 2009, 69, 365–372 CrossRef CAS.
- L. A. Utracki, Polymer blends handbook, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2002 Search PubMed.
- C. L. Tucker and P. Moldenaers, Microstructural evolution in polymer blends, Annu. Rev. Fluid Mech., 2002, 34, 177–210 CrossRef.
- J. Brame, Q. Li and P. J. Alvarez, Nanotechnology-enabled water treatment and reuse: emerging opportunities and challenges for developing countries, Trends Food Sci. Technol., 2011, 22, 618–624 CrossRef CAS.
- J. Huang, G. Arthanareeswaran and K. Zhang, Effect of silver loaded sodium zirconium phosphate (nanoAgZ) nanoparticles incorporation on PES membrane performance, Desalination, 2012, 285, 100–107 CrossRef CAS.
- D. Y. Koseoglu-Imer, B. Kose, M. Altinbas and I. Koyuncu, The production of polysulfone (PS) membrane with silver nanoparticles (AgNP): physical properties, filtration performances, and biofouling resistances of membranes, J. Membr. Sci., 2013, 428, 620–628 CrossRef CAS.
- A. Agarwal, T. L. Weis, M. J. Schurr, N. G. Faith, C. J. Czuprynski, J. F. McAnulty, C. J. Murphy and N. L. Abbott, Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells, Biomaterials, 2010, 31, 680–690 CrossRef CAS PubMed.
- Q. Bao, D. Zhang and P. Qi, Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection, J. Colloid Interface Sci., 2011, 360, 463–470 CrossRef CAS PubMed.
- J. Chen, H. Peng, X. Wang, F. Shao, Z. Yuan and H. Han, Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation, Nanoscale, 2014, 6, 1879–1889 RSC.
- H. N. Abdelhamid, M. S. Khan and H.-F. Wu, Graphene oxide as a nanocarrier for gramicidin (GOGD) for high antibacterial performance, RSC Adv., 2014, 4, 50035–50046 RSC.
- H. Zhang, D. Yee and C. Wang, Quantum dots for cancer diagnosis and therapy: biological and clinical perspectives, Nanomedicine, 2008, 3, 83–91 CrossRef CAS PubMed.
- T. Maneerung, S. Tokura and R. Rujiravanit, Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing, Carbohydr. Polym., 2008, 72, 43–51 CrossRef CAS.
- M. Toroghi, A. Raisi and A. Aroujalian, Preparation and characterization of polyethersulfone/silver nanocomposite ultrafiltration membrane for antibacterial applications, Polym. Adv. Technol., 2014, 25, 711–722 CrossRef CAS.
- J. Ma, J. Zhang, Z. Xiong, Y. Yong and X. Zhao, Preparation, characterization and antibacterial properties of silver-modified graphene oxide, J. Mater. Chem., 2011, 21, 3350–3352 RSC.
- Y. Shen, T. Jing, W. Ren, J. Zhang, Z.-G. Jiang, Z.-Z. Yu and A. Dasari, Chemical and thermal reduction of graphene oxide and its electrically conductive polylactic acid nanocomposites, Compos. Sci. Technol., 2012, 72, 1430–1435 CrossRef CAS.
- C. Xu and X. Wang, Fabrication of Flexible Metal-Nanoparticle Films Using Graphene Oxide Sheets as Substrates, Small, 2009, 5, 2212–2217 CrossRef CAS PubMed.
- C.-N. Lok, C.-M. Ho, R. Chen, Q.-Y. He, W.-Y. Yu, H. Sun, P.-H. Tam, J.-F. Chiu and C.-M. Che, Silver nanoparticles: partial oxidation and antibacterial activities, J. Biol. Inorg. Chem., 2007, 12, 527–534 CrossRef CAS PubMed.
- G. Goncalves, P. A. A. P. Marques, C. M. Granadeiro, H. I. S. Nogueira, M. K. Singh and J. Grácio, Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth, Chem. Mater., 2009, 21, 4796–4802 CrossRef CAS.
- Y. Si and E. T. Samulski, Exfoliated Graphene Separated by Platinum Nanoparticles, Chem. Mater., 2008, 20, 6792–6797 CrossRef CAS.
- S. Liu, T. H. Zeng, M. Hofmann, E. Burcombe, J. Wei, R. Jiang, J. Kong and Y. Chen, Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress, ACS Nano, 2011, 5, 6971–6980 CrossRef CAS PubMed.
- S. Jose, A. S. Aprem, B. Francis, M. C. Chandy, P. Werner, V. Alstaedt and S. Thomas, Phase morphology, crystallisation behaviour and mechanical properties of isotactic polypropylene/high density polyethylene blends, Eur. Polym. J., 2004, 40, 2105–2115 CrossRef CAS.
- P. K. S. Mural, G. Madras and S. Bose, Positive temperature coefficient and structural relaxations in selectively localized MWNTs in PE/PEO blends, RSC Adv., 2014, 4, 4943–4954 RSC.
- P. K. S. Mural, M. S. Rana, G. Madras and S. Bose, PE/PEO blends compatibilized by PE brush immobilized on MWNTs: improved interfacial and structural properties, RSC Adv., 2014, 4, 16250–16259 RSC.
- S. Bose, C. Özdilek, J. Leys, J. W. Seo, M. Wübbenhorst, J. Vermant and P. Moldenaers, Phase Separation as a Tool to Control Dispersion of Multiwall Carbon Nanotubes in Polymeric Blends, ACS Appl. Mater. Interfaces, 2010, 2, 800–807 CAS.
- G. Vleminckx, S. Bose, J. Leys, J. Vermant, M. Wübbenhorst, A. A. Abdala, C. Macosko and P. Moldenaers, Effect of Thermally Reduced Graphene Sheets on the Phase Behavior, Morphology, and Electrical Conductivity in Poly[(α-Methyl Styrene)-co-(Acrylonitrile)/Poly(Methyl-Methacrylate) Blends, ACS Appl. Mater. Interfaces, 2011, 3, 3172–3180 CAS.
- C. Marambio-Jones and E. M. Hoek, A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- A. Muñoz-Bonilla and M. Fernández-García, Polymeric materials with antimicrobial activity, Prog. Polym. Sci., 2012, 37, 281–339 CrossRef.
- M. Sharma, G. Madras and S. Bose, Unique nanoporous antibacterial membranes derived through crystallization induced phase separation in PVDF/PMMA blends, J. Mater. Chem. A, 2015, 3, 5991–6003 CAS.
- Y. Zhu, M. D. Stoller, W. Cai, A. Velamakanni, R. D. Piner, D. Chen and R. S. Ruoff, Exfoliation of Graphite Oxide in Propylene Carbonate and Thermal Reduction of the Resulting Graphene Oxide Platelets, ACS Nano, 2010, 4, 1227–1233 CrossRef CAS PubMed.
- C. Baker, A. Pradhan, L. Pakstis, D. J. Pochan and S. I. Shah, Synthesis and antibacterial properties of silver nanoparticles, J. Nanosci. Nanotechnol., 2005, 5, 244–249 CrossRef CAS PubMed.
- J. S. Taurozzi, H. Arul, V. Z. Bosak, A. F. Burban, T. C. Voice, M. L. Bruening and V. V. Tarabara, Effect of filler incorporation route on the properties of polysulfone–silver nanocomposite membranes of different porosities, J. Membr. Sci., 2008, 325, 58–68 CrossRef CAS.
- M. J. Hajipour, K. M. Fromm, A. Akbar Ashkarran, D. Jimenez de Aberasturi, I. R. d. Larramendi, T. Rojo, V. Serpooshan, W. J. Parak and M. Mahmoudi, Antibacterial properties of nanoparticles, Trends Biotechnol., 2012, 30, 499–511 CrossRef CAS PubMed.
- C. D. Vecitis, K. R. Zodrow, S. Kang and M. Elimelech, Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes, ACS Nano, 2010, 4, 5471–5479 CrossRef CAS PubMed.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, The bactericidal effect of silver nanoparticles, Nanotechnology, 2005, 16, 2346 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |