Supramolecular structures ranging from nano- to macro-scale with fluorescent and organic semiconducting properties†
Yanjun Gonga,
Qiongzheng Hub,
Ni Chenga,
Yanhui Bia,
Wenwen Xuac and
Li Yu*a
aKey Laboratory of Colloid and Interface Chemistry, Shandong University, Ministry of Education, Jinan 250100, P. R. China
bDepartment of Chemistry, University of Houston, Houston 77204, USA
cSchool of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, P. R. China
First published on 27th March 2015
Abstract
Supramolecular structures ranging from nano- to macro-scale are prepared by an ionic self-assembly (ISA) strategy with commercially available, low-cost dyes and surfactants, viz. Rhodamine 6G (R6G) and sodium bis(2-ethylhexylhexyl) sulfosuccinate (NaAOT). The organic small compounds are employed to fabricate fibers with a length up to two centimeters via ISA. They exhibit strong strength and high flexibility and could be bent without damaging the original shape. It is noteworthy that this supramolecular complex also displays both remarkable solid-state red light emission and organic semiconducting behavior. It is extremely rare that the looped nanochains comprised of organic nanoparticles are found during the formation process of the supramolecular structures. A possible mechanism for the formation of the macroscopic fibers is proposed. The meso- and macro-scale fibers can be widely used in the development of multi-scale materials with high optoelectronic performance.
Introduction
Supramolecular self-assembly1 offers a potent and versatile route to prepare well-defined, discrete supramolecular architectures from simple molecular components under thermodynamic equilibrium, e.g. organic luminescent materials,2–4 organogels,5,6 liquid-crystalline materials7 and biocompatible materials.8,9 However, these self-assembled complexes generally have local domain-like structures, and therefore show a poor macroscopic order. The reported self-assembly materials with ordered structures are usually in the nanometer and micrometer scale but rarely up to millimeter scale.10 Design and fabrication of supramolecular assembly with macro-scale size and superior properties are still a challenge.Faul's group reported a macroscopic order liquid-crystalline material blended with different molecular weights of polystyrenes.11 Perlmutter et al. constructed self-assembly of N-acetyl-capped β-peptides, resulting in the formation of nano- to macro-scale fiber.12 Ordered macroscopic materials are always comprised of various complex which in large have been prepared by complicate organic synthesis methods. Fabrication of macroscopic materials in high order is attractive via self-assembly of simple molecules in a facile way.
Dyes have advantages such as wide availability, diversity of functional groups, unique optical and electronic properties. Owing to these features, dye combined with oppositely charged surfactants are the ideal building blocks for ionic self-assembly (ISA).13–15 Herein, we employ the ISA approach to facilely prepare birefringent and fluorescent macro-scale fibers by using commercially available and low-cost dye and surfactants, namely Rhodamine 6G (R6G) and sodium bis(2-ethylhexylhexyl) sulfosuccinate (NaAOT). Chemical structures of R6G and NaAOT are shown in Fig. S1.† This method is simple and effective, which avoids complex organic synthesis and shows potential for the fabrication of highly ordered macroscopic materials that can be widely used in non-linear optical devices, optoelectronic materials and fast switching, etc.16–19
Experimental section
Materials
Rhodamine 6G (R6G) and sodium bis(2-ethylhexylhexyl) sulfosuccinate (NaAOT) were purchased from J&K Chemical Technology. Urea was purchased from J&K Chemical Technology. The water used was triply distilled and its specific conductance is 1.2 μS cm−1.Preparation of supramolecular structures
Aqueous solutions of NaAOT (10 mL, 0.5 mM) and R6G (10 mL, 0.5 mM) were mixed and stirred in a 50 mL flask at 25 °C. After additional stirring for 10 min, the mixture was placed in a thermostat at 25 °C. The obtained products were collected by filtration, washed three times with water to remove salts and possible precursors. The final products were dried under vacuum at 25 °C for 24 h.Characterizations of supramolecular structures
The sizes and morphologies of supramolecular materials were characterized by scanning electron microscopy (SEM, JEOL JSM-7600F) operated at 5.0 kV. The samples were prepared by placing a drop of supramolecular aggregates in water onto a silicon wafer.The nanostructures were characterized by transmission electron microscopy (TEM) (JEM-100CX II (JEOL). The morphologies of samples were also characterized by a polarized optical microscope (POM) equipped with a charge-coupled device camera (Panasonic Super Dynamic II WVCP460).
The 1H NMR spectra were recorded using a Bruker AV-300 NMR spectrometer with a pulse field gradient module (Z-axis) and a 5 mm NMR sample tube. The instrument was operated at a frequency of 300.13 MHz at 25 °C with tetramethylsilane as an internal reference. Deuterated dimethylsulfoxide (DMSO) was selected as the solvent.
AFM images were collected in air under ambient conditions using the tapping mode with a NanoscopeIII/Bioscope scanning probe microscope from digital instruments.
Small angle X-ray scattering (SAXS) measurements were performed using an Anton-paar SAX Sess mc2 system with a Ni-filtered Cu Kα radiation (1.54 Å) operated at 50 kV and 40 mA. The solid samples were placed in a stainless steel tank sealed by an aluminum foil. The distance from sample to detector was 264.5 mm.
Fourier transform IR (FTIR) spectra were recorded between 4000 and 400 cm−1 by using a VERTEX-70/70v FTIR spectrometer (Bruker Optics, Germany) on pressed thin KBr disks of samples.
UV/Vis spectra were measured in a quartz plate by using a HP 8453E instrument.
Cyclic voltammetric (CV) measurements were performed in a standard three-electrode cell, with Pt/C as the working electrode and Pt as auxiliary electrode and Ag/AgCl electrode (saturated KCl) as the reference electrode. Tetrabutylammonium perchlorate (0.1 M) was used as supporting electrolyte and ferrocene was used as internal reference electrode with different scanning rate of initial voltage: −1.9 V, segment: 2, sensitivity: 1 × 10−4 A/V.
Results and discussion
Fibers with the length of up to two centimeters are synthesized using the ISA strategy. The obtained fibers can bear the weight of two TEM copper grids (Fig. 1a), which indicates strong mechanical strength. In addition, the constructed macroscopic fibers could be bent and stretched without damaging the original shape (Fig. 1b), indicative of their good flexibility. The POM and OM images of the fibers are shown in Fig. 1c and d, respectively. The macroscopic fibers exhibit strong birefringence.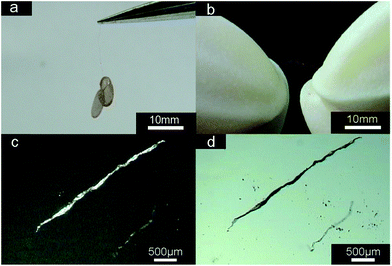 | ||
| Fig. 1 Macroscopic fibers (a and b), POM image (c), and OM image (d) of microscopic fibers formed by R6G and NaAOT in water. [R6G] = 0.25 mM, [NaAOT] = 0.25 mM. | ||
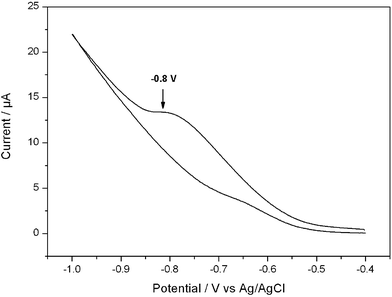
Beyond the optical and mechanical properties, we also studied semiconducting behavior of the fabricated microfibers. As depicted in Fig. 2, under the condition of scan rate of 50 mV s−1, the supramolecular complex exhibits an irreversible wave with Ered = −0.8. At the same time, the Eredox can also be detected at other different scan rate (Fig. S2†). Based on the reduction potential, we performed a series of calculation. The zero vacuum level of ferrocene was taken as 4.8 eV. The equations employed for the calculation of LUMO and HOMO energies are as follows.20,21
| ELUMO = −e(Eredox − Efer + 4.8) | (1) |
| EHOMO = ELUMO − hc/λoffset | (2) |
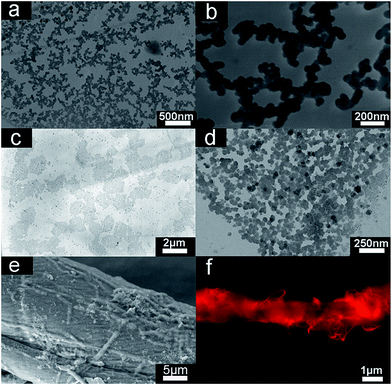
We found that R6G and NaAOT compounds could self-assemble into spherical particles after the mixture was incubated for 40 min due to the supramolecular amphiphilic feature of the introduced molecules in water. TEM images show the size of spherical particles ranges from 40 to 70 nm (Fig. 3a). AFM images display that the nanoparticle structures can be well-maintained on freshly mica or glass slides (Fig. S3†), indicative of their good stability on different substrates.25
To clarify the structure of nanoparticles, the composition of nanoparticles was examined firstly. All proton signals of R6G and NaAOT were obviously observed by 1H NMR spectra (Fig. S4†). The stoichiometry of these two compounds is confirmed to be a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio by comparing the peak integral intensities. SAXS diffractogram shows two scattering peaks of the extremely highly ordered supramolecular nanoparticles (Fig. S5†). The ratio of the scattering factor for the two peaks is 1
1 molar ratio by comparing the peak integral intensities. SAXS diffractogram shows two scattering peaks of the extremely highly ordered supramolecular nanoparticles (Fig. S5†). The ratio of the scattering factor for the two peaks is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, suggesting a typical lamellar structure of nanoparticles with an interplanar distance (d) of 2.18 nm. To further evaluate how these highly ordered nanoparticles are formed by the ISA strategy, interactions between R6G and NaAOT were also investigated. The electrostatic interaction was confirmed by FTIR pattern and Gaussian 09 program. It has been reported that pure NaAOT has a characteristic absorption band at 1050 cm−1 corresponding to the vibration of sulfonic group.26,27 As presented in Fig. S6,† for the complex, the absorption peak of sulfonic group shifts to 1028 cm−1, which indicates the existence of electrostatic interaction. Based on the above information, three possible positions of NaAOT were considered for the theoretical calculations (Fig. S7a†). All the optimized geometries (Fig. S7b†) were obtained by energy minimized molecular model using density functional theory (DFT) at the B3LYP/6-31G (d and p) level. The molecular arrangement of complex in the position 3 has the lowest interaction energy, which implies that it is the most optimized structure. To reveal the effect of hydrophobic interaction on the formation of nanoparticles, fluorescence spectroscopic investigation was carried out. As presented in Fig. S8,† a weak and broad emission band is observed around 660 nm for R6G, which can be ascribed to the excimer emission. Contrastingly, the emission spectrum of R6G–NaAOT complex shows an intense peak at about 650 nm. A rational explanation is that hydrophobic interaction of NaAOT destroys the close packing of R6G. UV/Vis spectra (Fig. S9†) confirm that π–π stacking interaction between R6G also has a strong influence on the formation of supramolecular aggregates. The maximum absorbance of R6G is located at 532 nm. However, when the complex is generated, the absorption band emerges at 552 nm. The redshift can be assigned to π–π stacking, which is typical for J-type aggregates.28 In such aggregates, the R6G is assembled in a “head to tail” arrangement, which can strongly enhance the fluorescence intensity.29
2, suggesting a typical lamellar structure of nanoparticles with an interplanar distance (d) of 2.18 nm. To further evaluate how these highly ordered nanoparticles are formed by the ISA strategy, interactions between R6G and NaAOT were also investigated. The electrostatic interaction was confirmed by FTIR pattern and Gaussian 09 program. It has been reported that pure NaAOT has a characteristic absorption band at 1050 cm−1 corresponding to the vibration of sulfonic group.26,27 As presented in Fig. S6,† for the complex, the absorption peak of sulfonic group shifts to 1028 cm−1, which indicates the existence of electrostatic interaction. Based on the above information, three possible positions of NaAOT were considered for the theoretical calculations (Fig. S7a†). All the optimized geometries (Fig. S7b†) were obtained by energy minimized molecular model using density functional theory (DFT) at the B3LYP/6-31G (d and p) level. The molecular arrangement of complex in the position 3 has the lowest interaction energy, which implies that it is the most optimized structure. To reveal the effect of hydrophobic interaction on the formation of nanoparticles, fluorescence spectroscopic investigation was carried out. As presented in Fig. S8,† a weak and broad emission band is observed around 660 nm for R6G, which can be ascribed to the excimer emission. Contrastingly, the emission spectrum of R6G–NaAOT complex shows an intense peak at about 650 nm. A rational explanation is that hydrophobic interaction of NaAOT destroys the close packing of R6G. UV/Vis spectra (Fig. S9†) confirm that π–π stacking interaction between R6G also has a strong influence on the formation of supramolecular aggregates. The maximum absorbance of R6G is located at 532 nm. However, when the complex is generated, the absorption band emerges at 552 nm. The redshift can be assigned to π–π stacking, which is typical for J-type aggregates.28 In such aggregates, the R6G is assembled in a “head to tail” arrangement, which can strongly enhance the fluorescence intensity.29
To gain an insight into the role of hydrogen-bonding interaction, urea (a concentration of 2 M) was added to examine whether the nanoparticles could still be formed.30 As reported,31–34 in aqueous solutions of urea, small amount of urea forms hydrogen bond with water. Most of the urea molecules are self-aggregated but the extent of aggregation is various at different concentrations. In some work urea is suggested to enhance the water structure as structure maker. Urea is also found that it can disrupt the natural water structure and is used as structure breaker. The hydrogen bonds between water–water molecules and solute–solute molecules can be destroyed by the introduction of the high concentration urea. In this work, 2 M urea can break the hydrogen bonds between R6G molecules in aqueous solution. As depicted in Fig. 3b, the nanoparticles were not obtained in the presence of urea. Instead, three-dimensional sponge-shape structures were formed. Therefore, hydrogen-bonding interactions play a critical role in the formation of supramolecular fibers. Furthermore, 1H NMR spectroscopy shows that there are no urea molecules in the R6G–NaAOT complex (Fig. S10†), implying that urea acts as a hydrogen-bonding breaker and does not directly constitute the formation of sponge-shape structures.
Fig. S5† also shows that in the complex, –NH groups of R6G are involved in strong hydrogen bonds, as clearly indicated by stretching vibrations at 3287 and 1730 cm−1. It is noted that ν(N–H) for free groups usually appears at 3370 and 1717 cm−1. This indicates H-bonding (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N) does exist during the formation of nanoparticles.35 To better understand the stacking structures and interactions, quantum simulation was performed by Gaussian 09 program with B3LYP/6-31G. The geometry optimization of R6G–NaAOT complex (Fig. S11†) displays that two adjacent RG6 have direct hydrogen bonding (N–H⋯O) and π–π stacking. The calculated distance and angle for H⋯O and N–H⋯O hydrogen bonds are 2.719 Å and 142°, respectively. Distance of the layers in the most optimal structure is 2.017 nm, which is consistent with the results of SAXS.36 Based on the above results, a possible structural model is proposed to explain the formation of nanoparticles, as illustrated in Scheme 1.
O⋯H–N) does exist during the formation of nanoparticles.35 To better understand the stacking structures and interactions, quantum simulation was performed by Gaussian 09 program with B3LYP/6-31G. The geometry optimization of R6G–NaAOT complex (Fig. S11†) displays that two adjacent RG6 have direct hydrogen bonding (N–H⋯O) and π–π stacking. The calculated distance and angle for H⋯O and N–H⋯O hydrogen bonds are 2.719 Å and 142°, respectively. Distance of the layers in the most optimal structure is 2.017 nm, which is consistent with the results of SAXS.36 Based on the above results, a possible structural model is proposed to explain the formation of nanoparticles, as illustrated in Scheme 1.
Fig. 4a and b display the representative TEM images of looped nanochains formed by nanoparticles ranging from 40–70 nm. The chains are one or two nanoparticles in width and comprised of bifurcated structures with outgrowths. An extended branched network is produced through the interconnection of the chains. As reported,37–40 self-assembled nanoparticles are inclined to form relatively linear aggregates due to hydrophobic interaction, electrostatic interaction, van der Waals forces and thermodynamic contributions. The present aggregations are equilibrium structures and cannot be explained by diffusion-limited growth mechanisms, which leads to structurally similar assembles under kinetic conduction. Hydrophobic interaction is a primary driving force in formation of chains. Based on the ISA strategy, a part of hydrophobic chain exists on the surface of nanoparticles. The hydrophobic chain can self-assemble to form linear particle chains with neighboring particles linked together through discrete hydrophobic “bonds” (Scheme 2).41 Electrostatic interactions play a large role in the self-assembly of particles. Van der Waals forces arise between all particles in the aqueous solution. The obtained particles with the diameter ranging from 40 to 70 nm are deemed to have strong Van der Waals forces and tend to fuse or to be coalescent in solution. Meanwhile aggregation can be prevented by protecting nanoparticles via “steric” and “electrostatic” stabilization. This creates a linear particle chains. At the same time, the formation of linear particle chains can decrease the surface energy. Reduction of surface energy in this way is different from that of Ostwald ripening.42–44
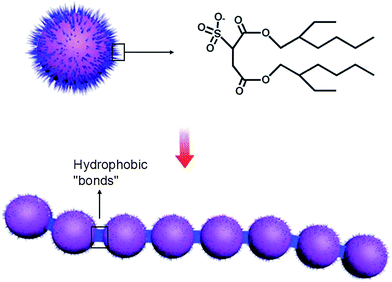 | ||
| Scheme 2 Nanoparticles with hydrophobic chains at their surface and nanoparticles can assemble into chain-like structures in the aqueous solution. | ||
Subsequently, the nanochains spontaneously form irregular aggregates, which could further be fused due to the hydrophobic and Van der Waals interaction and assembled into larger aggregates (Fig. 4c and d) that later self-organized to fibrils. The construction of the finally obtained rope-like microfibers from the fibrils (Fig. 4e) can be revealed by the small fibrils at the surface of the supramolecular self-assembled microfibers that exhibit strong fluorescence (Fig. 4f). The presence of microfibers suggests a mechanism of hierarchical self-assembly that follows a multistep “self-twining” process by means of the formation of consecutively higher order small fibrils.
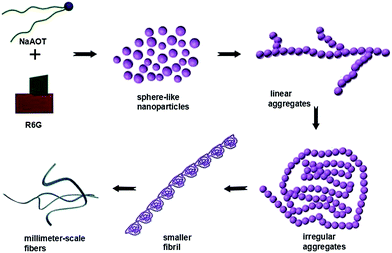
The formation mechanism of the macroscopic fibers is summarized as follows (Scheme 3): (1) sphere-like nanoparticles are primarily formed through noncovalent interactions, e.g. electrostatic and hydrophobic interactions, π–π stacking and hydrogen-bonding, between NaAOT and R6G compounds; (2) relatively linear aggregates emerge via the self-assembly of 0-D particles mainly due to hydrophobic interactions and these formed chains then interconnect to produce an extended branched network; (3) the chains are opt to aggregate preferentially to form micrometer-scale irregular aggregates with various shapes; (4) smaller fibrils are generated in solution from oriented connection of micrometer-scale irregular aggregates building blocks owing to the van der Waals forces. (5) Small fibrils self-assemble further to form fibers with millimeter scale.
Conclusion
In summary, we have demonstrated that the self-assembly of R6G and NaAOT can facilely lead to the formation of supramolecules with multi-scale morphologies, from nanoparticles to micro- and macro-scale fibers. The electrostatic, π–π stacking, H-bond and hydrophobic interactions are responsible for the fabrication of these supramolecular fibers. The obtained fibers up to two centimeters are strong and flexible. Furthermore, the supramolecular microfibers manifest superior fluorescent and birefringent properties. In addition, the materials also exhibit organic semiconducting behavior testified by the cyclic voltammograms. The inherent nature of this unique design together with ease of synthesis of the supramolecules provide potent new avenues for the development of novel multi-scale materials with superior properties., which may have potential application in optoelectronics and fast switching.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21373128), Scientific and Technological Projects of Shandong Province of China (no. 2014GGE27244), the Natural Science Foundation of Shandong Province of China (no. ZR2011BM017) and the Project of Sinopec (no. P13045).Notes and references
- F. Versluis, I. Tomatsu, S. Kehr, C. Fregonese, A. W. J. W. Tepper, M. C. A. Stuart, B. J. Ravoo, R. I. Koning and A. Kros, J. Am. Chem. Soc., 2009, 131, 13186–13187 CrossRef CAS PubMed.
- X. Zhang, J. Zou, K. Tamhane, F. F. Kobzeff and J. Fang, Small, 2010, 6, 217–220 CrossRef CAS PubMed.
- Z. Shen, T. Wang and M. Liu, Chem. Commun., 2014, 50, 2096–2099 RSC.
- X. Zhang, M. Mathew, A. J. Gesquiere and J. Fang, J. Mater. Chem., 2010, 20, 3716 RSC.
- X. Wang, P. Duan and M. Liu, Chem.–Eur. J., 2013, 19, 16072–16079 CrossRef CAS PubMed.
- Q. Jin, L. Zhang and M. Liu, Chem.–Eur. J., 2013, 19, 9234–9241 CrossRef CAS PubMed.
- J. A. Kelly, M. Giese, K. E. Shopsowitz, W. Y. Hamad and M. J. MacLachlan, Acc. Chem. Res., 2014, 47, 1088–1096 CrossRef CAS PubMed.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Science, 2001, 294, 1684–1688 CrossRef CAS PubMed.
- D. González-Rodríguez and A. P. H. J. Schenning, Chem. Mater., 2011, 23, 310–325 CrossRef.
- Z. Chen, Science, 1997, 277, 1248–1253 CrossRef CAS.
- A. Laiho, B. M. Smarsly, C. F. J. Faul and O. Ikkala, Adv. Funct. Mater., 2008, 18, 1890–1897 CrossRef CAS.
- M. P. Del Borgo, A. I. Mechler, D. Traore, C. Forsyth, J. A. Wilce, M. C. Wilce, M. I. Aguilar and P. Perlmutter, Angew. Chem., Int. Ed., 2013, 52, 8266–8270 CrossRef CAS PubMed.
- Y. Guan, Y. Zakrevskyy, J. Stumpe, M. Antonietti and C. F. J. Faul, Chem. Commun., 2003, 894–895 RSC.
- C. F. J. Faul and M. Antonietti, Adv. Mater., 2003, 15, 673–683 CrossRef CAS.
- C. F. J. Faul and M. Antonietti, Chem.–Eur. J., 2002, 8, 2764–2768 CrossRef CAS.
- G. Wu, P. Verwilst, K. Liu, M. Smet, C. F. J. Faul and X. Zhang, Chem. Sci., 2013, 4, 4486 RSC.
- K. Liu, Y. Yao, Y. Kang, Y. Liu, Y. Han, Y. Wang, Z. Li and X. Zhang, Sci. Rep., 2013, 3, 2372 Search PubMed.
- G. Wu, J. Thomas, M. Smet, Z. Wang and X. Zhang, Chem. Sci., 2014, 5, 3267–3274 RSC.
- Y. Q. Liu, T. Y. Wang, Y. Huan, Z. B. Liu, G. W. He and M. H. Liu, Adv. Mater., 2013, 25, 5875–5879 CrossRef CAS PubMed.
- M. R. Molla, D. Gehrig, L. Roy, V. Kamm, A. Paul, F. Laquai and S. Ghosh, Chem.–Eur. J., 2014, 20, 760–771 CrossRef CAS PubMed.
- S. Erten, S. Alp and S. Icli, J. Photochem. Photobiol., A, 2005, 175, 214–220 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino. G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian Inc., Wallingford, CT, 2009 Search PubMed.
- H. Kim, D. H. Lee and Y. A. Son, Textile Coloration and Finishing, J. Korean Soc. Dye. Finish., 2013, 25, 7–12 CrossRef PubMed.
- H. Luo, S. Chen, Z. Liu, C. Zhang, Z. Cai, X. Chen, G. Zhang, Y. Zhao, S. Decurtins, S. X. Liu and D. Zhang, Adv. Funct. Mater., 2014, 24, 4376–4376 CrossRef.
- C. Wang, Q. Chen, H. Xu, Z. Wang and X. Zhang, Adv. Mater., 2010, 22, 2553–2555 CrossRef CAS PubMed.
- P. D. Moran, G. A. Bowmaker, R. P. Cooney, J. R. Bartlett and J. L. Woolfrey, J. Mater. Chem., 1995, 5, 295–302 RSC.
- Q. Li, S. Weng, J. Wu and N. Zhou, J. Phys. Chem. B, 1998, 102, 3168–3174 CrossRef CAS.
- G. De Luca, A. Romeo, V. Villari, N. Micali, I. Foltran, E. Foresti, I. G. Lesci, N. Roveri, T. Zuccheri and L. M. Scolaro, J. Am. Chem. Soc., 2009, 131, 6920–6921 CrossRef CAS PubMed.
- M. Masia, J. Chem. Phys., 2008, 128, 184107 CrossRef PubMed.
- M. Zhao, Y. Zhao, L. Zheng and C. Dai, Chem.–Eur. J., 2013, 19, 1076–1081 CrossRef CAS PubMed.
- M. C. Stumpe and H. Grubmuller, J. Phys. Chem., 2007, 111, 6220 CrossRef CAS PubMed.
- M. C. Stumpe and H. Grubmuller, J. Am. Chem. Soc., 2007, 129, 16126–16131 CrossRef CAS PubMed.
- E. Finer, F. Franks and M. Tait, J. Am. Chem. Soc., 1972, 94(13), 4424–4429 CrossRef CAS.
- X. Hoccart and G. Turrell, J. Chem. Phys., 1993, 99(11), 8498–8503 CrossRef CAS PubMed.
- F. Camerel, G. Ulrich, J. Barbera and R. Ziessel, Chem.–Eur. J., 2007, 13, 2189–2200 CrossRef CAS PubMed.
- I. Ramakanth, N. Ramesh and A. Patnaik, J. Mater. Chem., 2012, 22, 17842 RSC.
- Z. Tang, N. A. Kotov and M. Giersig, Science, 2002, 297, 237–240 CrossRef CAS PubMed.
- H. Jia, X. Bai, N. Li, L. Yu and L. Zheng, CrystEngComm, 2011, 13, 6179 RSC.
- Y. Wang, Y. Wang and D. R. Breed, Nature, 2012, 491(7422), 51–55 CrossRef CAS PubMed.
- C. Pacholski, A. Kornowski and H. Weller, Angew. Chem., Int. Ed. Engl., 2002, 41, 1188–1191 CrossRef CAS.
- H. Y. lee, S. H. Shin, A. M. Drews, A. M. Chirsan, S. A. Lewis and K. J. M. Bishop, ACS Nano, 2014, 8, 9979–9987 CrossRef CAS PubMed.
- W. Lu, P. Gao, W. B. Jian, Z. L. Wang and J. Fang, J. Am. Chem. Soc., 2004, 126, 14816–14821 CrossRef CAS PubMed.
- R. L. Penn and J. F. Banfield, Geochim. Cosmochim. Acta, 1999, 63, 1549–1557 CrossRef CAS.
- S. Mahesh, A. Gopal, R. Thirumalai and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 7227–7230 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02256h |
| This journal is © The Royal Society of Chemistry 2015 |