Luciferase gene-loaded CS-Qdots as self-illuminating probes for specific hepatoma imaging
Chenyan Yuana,
Ling Wang†
ab,
Yanli Ana,
Guoqiu Wua and
Dongsheng Zhang*bc
aZhong Da Hospital, Medical School, Southeast University, No. 87 Dingjiaqiao, 210009 Nanjing, China
bMedical School, Southeast University, No. 87 Dingjiaqiao, 210009 Nanjing, China. E-mail: zdszds1222@163.com; Fax: +86-83272502; Tel: +86-13705177670
cJiangsu Key Laboratory For Biomaterials and Devices, No. 87 Dingjiaqiao, 210009 Nanjing, China
First published on 17th March 2015
Abstract
Chitosan encapsulated quantum dots (CS-Qdots) exhibit fascinating optical properties and can efficiently deliver genes into cells in a visualized process. By using CS-Qdots as gene carriers, specific hepatocellular carcinoma (HCC) expressed firefly luciferase genes (p[HRE]AFP-luc) were transfected into HCC cells for hepatoma bioluminescence imaging. The results obtained in this study show that nanocarrier CS-Qdots can be excited by the luciferase coded in the genes delivered into the cells. The maximum emission wavelength of the bioluminescence red-shifted from 560 nm to 630 nm. The excitation of CS-Qdots by bioluminescence occurs at the macroscopic scale and is independent of covalent bond. The luciferase gene-loaded CS-Qdots can act as wavelength-tunable self-illuminating probes thus holding potential for improved tumor optical molecular imaging.
1. Introduction
Optical imaging has emerged as a powerful technique in biomedical imaging because of its high sensitivity and low cost.1 The use of fluorescent imaging probes such as quantum dots presents challenges in vivo due to the requirement of an external light which produces strong autofluorescence from living tissues, thus compromising the imaging signal to noise ratio. In addition, the scattering and absorption of optical photons in living tissues significantly reduces the sensitivity and specificity of fluorescence imaging. The most widely used strategies attempt to solve these problems using near-infrared (NIR) imaging probes to reduce the autofluorescence, and self-illuminating imaging probes which do not depend on the excitation light and completely eliminate autofluorescence effects.2–5Quantum dots are often used as self-illuminating probes for their fascinating optical properties such as tunable photoluminescence, photochemical stability, high brightness and narrow emission bandwidth.6,7 Self-illuminating quantum dots absorb energy from a donor (usually an enzyme that emits light during the catalytic oxidation of its substrate, such as luciferase) and emit light without external excitation. In most cases, the establishment of self-illuminating quantum dot probes is based on bioluminescence resonance energy transfer (BRET), which is a non-radiative process for transferring energy. Two fundamental criteria must be satisfied: first, the emission spectrum of the donor must overlap the excitation spectrum of the acceptor; and second, these moieties must be in proximity (<10 nm).8–12 All luciferases are light-generating proteins coded by genes that can be selectively delivered to certain tissues or tumors, and are widely used in molecular and cell biology research applications, such as monitoring the expression of genes and specific tumor imaging.13 In BRET systems, luciferase proteins often bind quantum dots chemically, which limits the application range of self-illuminating quantum dots. Dragavon et al. first reported that Qdots could be activated by bioluminescence through a traditional radiative process which is independent on the covalent binding of luciferase and the Qdots.14 They defined the phenomenon as “fluorescence by unbound excitation from luminescence” (FUEL), a method that utilizes the conventional epifluorescence phenomenon. In the FUEL process, extremely low levels of unfocused, radiating bioluminescence can be used as an epifluorescent illumination light source. These interesting findings imply that quantum dots could be activated by endogenous luciferase expressed in specific tumor cells.
Targeted gene delivery allows for tumor bioluminescence imaging by using tumor- or tissue-specific promoters to restrict the expression of light-producing genes (such as firefly luciferase genes) in tumors and their metastases.15,16 In the current study, firefly luciferase genes induced by AFP promoter and hypoxia enhancement were prepared as we have previously reported, and the genes were delivered into cells by chitosan modified CdTe quantum dots (CS-Qdots) to establish a specific hepatoma imaging system. The AFP (α-fetoprotein) promoter is one of the most specific promoters which regulates downstream gene expression only in hepatocellular carcinoma (HCC) cells. The specificity and efficiency of gene expression in HCC cells driven by the AFP promoter and hypoxia enhancement have been validated by many studies.17–21 Chitosan encapsulated quantum dots as gene vectors provide a traceable delivering system, for example Tan et al. delivered HER2 siRNA into cells and achieved desirable silencing effects on the HER2 gene via RNA interference.22 Our interest focuses on whether CS-Qdots can act as gene carriers and self-illuminating probes simultaneously. There is a spectral overlap between the emission spectrum of bioluminescence and the absorption spectrum of CS-Qdots in our study. The emission peak of firefly luciferase is at about 560 nm, and the emission peak of the CS-Qdots is at 630 nm. If the CS-Qdots can be activated, the emission peak of bioluminescence should red-shift from 560 nm to 630 nm. The aim of this study is to provide experimental evidence to test whether gene carrier CS-Qdots can be excited by luciferase generated specifically in HCC cells. Fig. 1 is the schematic representation of gene-loaded nanoparticles based on quantum dots excited by luciferase generated intracellularly.
 | ||
| Fig. 1 Schematic representation of quantum dot based nanoparticles excited by luciferase generated intracellularly. | ||
2. Materials and methods
2.1. Main apparatus and materials
Transmission electron microscopy (TEM) was carried out on a JEM-200CX model (JEOL, Japan) and high resolution transmission electron microscopy (HRTEM) images were obtained with a JEM-2010 UHR model (JEOL, Japan). FTIR spectra were collected using a NEXUS870 spectrometer (NICOLET, USA). The ZetaPlus particle sizing analyzer used for the nanoparticles was purchased from Brookhaven instruments, USA. Bioluminescence and fluorescence imaging was carried out using an IVIS spectrum whole animal imaging system (Xenogen Corporation, Caliper Life Sciences, USA).Tellurium powder, NaBH4, CdCl2, 3-mercaptopropionic acid (MPA), EDC (N-ethyl-N-3-dimethylaminopropyl carbodiimide) and chitosan (deacetylation degree ≥ 95%) were all purchased from Sigma (USA). All the reagents used were of analytical grade.
Human HCC line HepG2 (AFP-positive) was maintained in Roswell Park Memorial Institute 1640 (RPMI 1640) supplemented with 10% fetal calf serum and incubated in a humidified 5% CO2 atmosphere at 37 °C.
2.2. Preparation of CdTe quantum dots (Qdots)
2.3. Preparation of chitosan (CS) encapsulated CdTe quantum dots (CS-Qdots)
A chitosan (CS) solution was prepared by dissolving 0.5 g of CS in 10 ml acetic acid solution (0.2 M). Then, the chitosan solution was diluted 100 times with pure water. The CdTe quantum dot solution was added to the diluted CS solution while stirring, then 0.5 ml of a EDC aqueous solution (0.5% w/v) was added. The reaction system was left overnight at room temperature. A CS encapsulated CdTe quantum dot colloid was obtained.2.4. Characterization of CdTe Qds and CS-Qdots
The morphology of CdTe Qdots was examined using TEM and HRTEM. The morphology of CS-Qdots was observed by TEM. UV-vis spectroscopy measurements of CS-Qdots were conducted using a fluorospectrophotometer (LS50B, PerkinElmer, USA). FTIR spectra of the CdTe and CS-Qdots were obtained on a FTIR spectrometer. The surface charge of CdTe Qdots (in pure water, 25 °C), CS-Qdots and DNA–CS-Qdots complexes at the relative ratio of 10 (in HEPES buffer of pH 6.8, 25 °C) were measured by a zeta plus particle sizing analyzer.2.5. Cellular uptake of CS-Qdots and imaging by confocal laser microscopy
The 5 × 104 per well HepG2 cells were seeded on a 6-well plate at 37 °C for 24 h. Then, CS-Qdots were added into the cell dished at 100 μg ml−1. After incubation for 12 h, the cells were washed with PBS three times to remove free CS-Qdots. The cell nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI, Sigma, USA) before detection with a confocal laser microscope (FluoView FV1000, Olympus, Japan).2.6. Gel retardation assay
The p[HRE]AFP-luc plasmids which specifically express firefly luciferase in HCC cells were prepared as we have previously reported.17 Plasmid–CS-Qdot complexes at various NP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA weight ratios were prepared and incubated for 30 min. Then the complexes were added to the wells (10 μg DNA per lane) of a 0.8% agarose gel, and run at 100 V for 30 h. Samples of free DNA, free CS-Qdots and CdTe Qdots (without chitosan) were added as controls. Images were obtained on a gel imaging analysis system-JS-680D (Shang Hai Peiqing Science and Technology Corporation, China).
DNA weight ratios were prepared and incubated for 30 min. Then the complexes were added to the wells (10 μg DNA per lane) of a 0.8% agarose gel, and run at 100 V for 30 h. Samples of free DNA, free CS-Qdots and CdTe Qdots (without chitosan) were added as controls. Images were obtained on a gel imaging analysis system-JS-680D (Shang Hai Peiqing Science and Technology Corporation, China).
2.7. pEGFP-C1 delivery into HepG2 cells by CS-Qdots
HepG2 cells were seeded on a 6-well plate 24 h prior to treatments. The GFP (green fluorescent protein) expression vector pEGFP-C1 (Sangon Biotech, China) and CS-Qdots were diluted in RPMI 1640 (pH 6.8) separately, then gently mixed together by pipetting, and incubated for 30 min at room temperature. The mass ratio of plasmid and CS-Qdots nanoparticles in the transfection complexes was 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NP
1 (NP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA weight ratio). Then, the medium was removed and the cells were washed by PBS (pH 6.8) for 3 times before the dropwise addition of the particle–DNA transfection complexes. The transfection complexes were replaced with fresh medium at 12 h post addition. Then ultra structure of the cells and distribution of CS-Qdots in the cells was observed by TEM. The expression of GFP and CS-Qdots internalized by cells was directly visualized under a confocal laser microscope (FluoView FV1000, Olympus, Japan) at 48 h post transfection.
DNA weight ratio). Then, the medium was removed and the cells were washed by PBS (pH 6.8) for 3 times before the dropwise addition of the particle–DNA transfection complexes. The transfection complexes were replaced with fresh medium at 12 h post addition. Then ultra structure of the cells and distribution of CS-Qdots in the cells was observed by TEM. The expression of GFP and CS-Qdots internalized by cells was directly visualized under a confocal laser microscope (FluoView FV1000, Olympus, Japan) at 48 h post transfection.
2.8. Establishment of HCC xenografts nude mice model
Healthy male BALC/c nude mice, aged 6 weeks, weighing 18–20 g were used for the experiments. The animal experiments were approved by the Animal Care Committee of Jiangsu Province and were performed in accordance with the institutional guidelines. All the mice were maintained in the Sterile Barrier System of the Medical School, Southeast University, China. Exponentially growing HepG2 cells (2 × 106) were injected subcutaneously in the limb of the mice to establish the hepatoma xenografts models.2.9. Bioluminescence imaging in vitro and in vivo
The mouse bearing the xenograft was intratumorally injected with 100 μl DNA–CS-Qdots transfection complexes, which consisted of 120 μg CS-Qdots and 10 μg p[HRE]AFP-luc DNA, prepared 30 min prior to injection. At 72 hours post injection, the mouse was injected intraperitoneally with a D-luciferin sodium salt solution (15 mg ml−1, 10 μl g−1 weight, Gold biotechnology, USA) and the bioluminescence and fluorescence signals were acquired after 10 min. The bioluminescence signal was detected at four wave bands (560 ± 10 nm, 600 ± 10 nm, 620 ± 10 nm and 640 ± 10 nm), 3 times for each band-pass filter. The mean values were calculated and the luminous intensity–wavelength curves were then plotted.
3. Results
3.1. Characterizations of CS-Qdots
The morphology and crystal structure of CS-Qdots were studied by TEM and HRTEM as shown in Fig. 2A, the size of the fluorescent crystals is about 5 nm. The CS-Qdots were found to have spherical morphology with a good dispersion in the TEM image. The mean particle size is about 25–30 nm (Fig. 2B). When CdTe quantum dots were encapsulated by chitosan, the characteristic peaks of chitosan (–NH2 vibration, C–H stretching vibration) were observed on the FTIR spectra (Fig. 2F). The surface charge of CdTe Qdots is −16.31 ± 0.91 mV (Fig. 2C) but shifted to a positive value of 28.02 ± 1.15 mV for CS-Qdots (Fig. 2D). The zeta potential of the DNA–CS-Qdots complex at a relative ratio of 10 (CS-Qdots![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA) is 10.02 ± 1.21 mV (Fig. 2E).
DNA) is 10.02 ± 1.21 mV (Fig. 2E).
3.2. CS-Qdots internalized by cells
Due to their small size and positive surface charge, CS-Qdots can be readily internalized by cells. As shown in Fig. 3, cells containing CS-Qdots were observed on a confocal laser microscope and the images revealed that the particles were concentrated in the cytoplasm of the cells (the red colour belongs to CS-Qdots and the blue colour arises from nuclei stained with DAPI).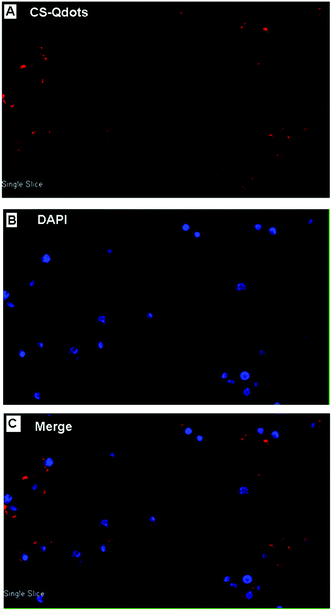 | ||
| Fig. 3 Confocal laser microscopic views of HepG2 cells incubated with CS-Qdots (100 μg ml−1) for 12 h. (A) Excitation of CS-Qdots. (B) Excitation of DAPI. (C) Merged image. | ||
3.3. Gel retardation assay
Firefly luciferase plasmids (p[HRE]AFP-luc) specifically expressed in hepatocellular carcinoma cells were prepared as we have previously reported.17 The schematic structure is shown in Fig. 4. The plasmid binding affinity of CS-Qdots was tested at various weight ratios by agarose gel electrophoresis. As shown in Fig. 5, complete retardation was observed for particle–DNA weight ratios over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, for which the DNA is well packed in the gene–CS-Qdots complexes with a positive surface charge.
1, for which the DNA is well packed in the gene–CS-Qdots complexes with a positive surface charge.
 | ||
Fig. 5 Agarose gel electrophoresis tests for DNA retention in p[HRE]AFP-luc loaded CS-Qdots prepared at various CS-Qdots nanoparticle![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DNA weight ratios. DNA weight ratios. | ||
3.4. GFP gene transfected into HepG2 cells by using CS-Qdots
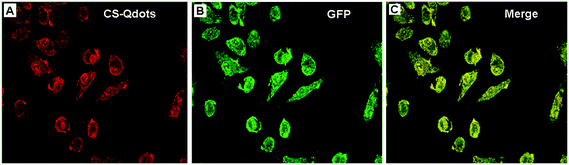
Reporter gene pEGFP-C1 (expressing GFP) was used to evaluate the transfection efficiency of CS-Qdots. At 12 h post transfection, the cells’ ultra-structure was observed by TEM. As shown in Fig. 6, the HepG2 cell is enveloping clusters of CS-Qdots (indicated by the black circle and arrows) while some nanoparticles have already been internalized in the cytoplasm (indicated by the white arrow).Two days after transfection, HepG2 cells were observed on a confocal laser microscopy. As shown in Fig. 7, the red colour represents the CS-Qdots as the gene are internalized into the cells, and the green colour represents the GFP expressed in the cells. The results indicate a high level expression of GFP genes which were delivered into the cells by CS-Qdots.
3.5. CS-Qdots excited by bioluminescence in vitro (extracellular)
The experiment was designed to test our hypothesis that CS-Qdots could be directly excited by bioluminescent light from the cells over macroscopic distances. HepG2 cells were seeded on two 24-well plates where 8 wells containing cells surrounded a blank well as shown in Fig. 8A. Then, all the cells were transfected with p[HRE]AFP-luc by Lipofectamine 2000 48 h prior to imaging. Before acquiring bioluminescence and fluorescence images, 1 ml CS-Qdots (0.1 mg ml−1) were added to one of the blank wells surrounded by wells containing cells (Fig. 8B and C). The corresponding wells on the other 24-well plates were left blank (Fig. 8A). Bioluminescence imaging of the cells transfected with p[HRE]AFP-luc was observed as shown in Fig. 8A. For the plate with CS-Qdots, the bioluminescence signal of the cells was quenched and the luminescence signal of CS-Qdots was detectable (Fig. 8C). This result implies that energy was transferred from the bioluminescent cells to the CS-Qdots. The energy transfer mechanism is a simple excitation–emission process which Dragavon et al. characterized as the FUEL effect.143.6. Detection of the bioluminescence signal at different wave bands in vitro
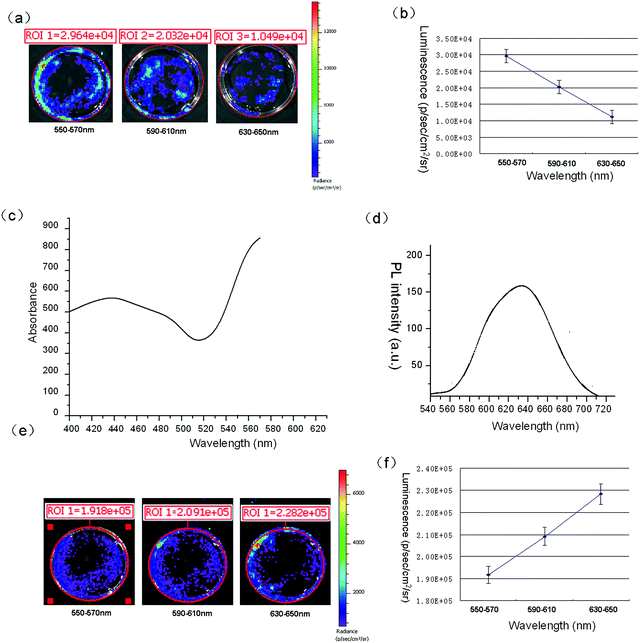
To test whether CS-Qdots could act as gene carriers and self-illuminating probes simultaneously, it was important to detect the wavelength of maximal bioluminescence of the cells transfected with p[HRE]AFP-luc using CS-Qdots as gene carriers. An IVIS spectrum whole animal imaging system was used to acquire the bioluminescence signal at different wave bands. As it can be seen in Fig. 9A, HepG2 cells transfected with p[HRE]AFP-luc by Lipofectamine 2000 and their bioluminescence signals were measured with three filters: 550–570 nm, 590–610 nm and 630–650 nm. Then, the signal–wavelength curve was plotted, from which it is easy to see that the maximum signal of bioluminescence is at 550–570 nm (Fig. 9B). The absorption spectrum of CS-Qdots was measured as shown in Fig. 9C. The peak of bioluminescent light emission lies in a region of the wavelength spectrum where the CS-Qdots display a strong absorbance peak. If the CS-Qdots are excited by the luciferase generated in the cells and the luminescence signal is detectable, the emission peak of the bioluminescence should red-shift from 560 nm to 630 nm, consistent with the emission spectrum of CS-Qdots (Fig. 9D). The bioluminescence of cells transfected with p[HRE]AFP-luc by CS-Qdots was measured at the same three filters (Fig. 9E). As shown in the signal–wavelength curve in Fig. 9F, the maximal luminescence signal is at 630–650 nm. The results demonstrate that CS-Qdots can be excited by bioluminescence intracellularly.3.7. CS-Qdots excited by bioluminescence in vivo
The p[HRE]AFP-luc/CS-Qdots transfection complexes were injected into the tumors of the HCC xenografts nude mice, and the bioluminescence signal was detected 72 hours later. Four filters were used (550–570 nm, 590–610 nm, 610–630 nm and 630–650 nm) to detect the wavelength of the maximal signal of bioluminescence, as shown in Fig. 10A. The peak of light emission was at 630–650 nm, the same as in the in vitro experiments. The bioluminescence and fluorescence images were acquired (Fig. 10B and C) and merged in Fig. 10D.4. Discussion
Chitosan is a polycationic natural polysaccharide which has been widely used in pharmaceuticals as drug or gene carriers and other delivery systems.23,24 Chitosan has basic amine groups on its glycosidic residues which contribute to the positive charge of the natural polysaccharide.25 Chitosan encapsulated quantum dots (CS-Qdots) as multifunctional nanomaterials with unique optical properties combine the advantages of quantum dots and chitosan, which could efficiently deliver genes into cells and provide a traceable delivery system in vitro and in vivo. CS-Qdots bear several positive charges that facilitate interactions with negatively charged cell membranes and enable efficient cellular uptake via endocytosis. The evidence from the experiments show that when specific hepatoma-expressed luciferase genes (p[HRE]AFP-luc) are delivered into HCC cells by CS-Qdots, the fluorescent nanoparticles are excited by the luciferase generated in the cells.Dragavon et al.14 have reported that quantum dots can be excited by luminescent bacteria in vivo and in vitro by a mechanism named “fluorescence by unbound excitation from luminescence” (FUEL), a method that utilizes the conventional epifluorescence phenomenon. The results from our experiments indicate that CS-Qdots can also be excited by luminescent cells transfected with luciferase genes through FUEL. FUEL is different from reported nanometer scale molecular proximity methods such as BRET, since the energy transfer of FUEL from the donor (bioluminescence of the firefly luciferase) to the acceptor (CS-Qdots) is in the macroscopic scale. When CS-Qdots are surrounded by luminescent cells, they are excited and a luminescence signal from them is detectable, although the bioluminescence signal of the cells is quenched. The results from the experiments provide direct evidence that CS-Qdots can be excited by bioluminescence in the macroscopic scale. In addition, the excitation of the CS-Qdots in our study is not the same as in Dragavon’s, since the luciferase light source is coded in the genes transfected into the cells while in Dragavon’s study the quantum dots were excited by bioluminescent bacterias expressing the lux operon. When HCC cells are transfected with plasmids p[HRE]AFP-luc by CS-Qdots in vivo and in vitro, emission spectrum analysis of the cells indicate that the emission peak of the bioluminescence red-shifts from 560 nm to 630 nm, consistent with the spectrum of CS-Qdots. Therefore, luciferase gene loaded CS-Qdots have the potential to detect HCC cells as self-illuminating probes.
Self-illuminating nanoparticles are ideal fluorescence probes which can emit light without external excitation and avoid autofluorescence completely. The luciferase gene loaded CS-Qdots are different from most self-illuminating quantum dots whose design is based on the principle of bioluminescence resonance energy transfer (BRET).26–28 The main disadvantage of BRET self-illuminating quantum dot imaging is the lack of tissue or cell specificity. One of the requirements needed for BRET quantum dots is that the distance between the donor and the acceptor must be less than 10 nm, so the BRET self-illuminating quantum dots are usually attached to the luciferase proteins by covalent bonds. In the current study, firefly luciferase, which acts as an epifluorescent illumination light source, is generated in the cells. Compared to immobilized luciferase, gene coded luciferase proteins can be selectively delivered into certain tissues or tumors by targeted gene delivery systems.
The bioluminescence is due to light emission during the chemical reaction between the luciferase protein and its substrate, resulting in non-invasive imaging in healthy cells and living animals.29 Tumor-specific luciferase gene expression can be driven by tissue- or tumor-specific promoters, such as the α-fetoprotein (AFP) promoter for hepatocellular carcinoma (HCC). AFP gene is expressed in fetal or embryonic livers and inactivated after birth. However, in HCC cells, AFP gene is re-activated and re-expressed.30 Luciferase genes induced by AFP promoter are expressed in HCC cells by a restricting sequence-specific mechanism. The evidence from our experiments shows that the CS-Qdots nanoparticles can be excited by HCC-targeted expressed firefly luciferase in vitro and in vivo. The CS-Qdots excited by bioluminescence through FUEL are self-illuminating probes with strict tumor specificity.
It is noticeable that the self-illuminating CS-Qdot probes are simultaneously gene carriers, which deliver HCC-specific luciferase plasmids (p[HRE]AFP-luc) into hepatoma cells. The promoter gene of the plasmids can be easily replaced to track down other cancers in the body, such as the PSA promoter used for prostate carcinoma31 or the chromogranin A promoter for neuroendocrine tumors, etc.32 Moreover, the maximum emission of the versatile quantum dots is tunable based on the particle size of the crystal,33 and so the light emitting CS-Qdot wavelength is also tunable. The luciferase gene-loaded CS-Qdots in the current study, which combine the advantages of quantum dots (such as superior brightness and photo stability, tunable emission, etc.) as well as the high sensitivity and specificity of bioluminescence imaging, can act as attractive self-illuminating probes holding potential for improved deep tumor in vivo imaging.
5. Conclusions
When hepatoma cell expressed firefly luciferase genes (p[HRE]AFP-luc) are delivered into HCC cells by nanoparticles based on quantum dots (CS-Qdots), the nanoparticles can be excited by the bioluminescence of the transfected cells. The emission peak of the bioluminescence signal red-shifts from 560 nm to 630 nm. The energy transfer from the luciferase generated in the transfected cells to the CS-Qdots is achieved by “fluorescence by unbound excitation from luminescence” (FUEL). The evidence from the experiments indicates that luciferase gene loaded CS-Qdots nanoparticles can act as self-illuminating probes for improved specific tumor imaging.Disclosure of interest
The authors declare no competing financial interest or interest otherwise.Acknowledgements
This work was supported by the Chinese National 863 Plan (2007AA03Z356), the Chinese National Natural Science Foundation (81301270 and 81171452). And I would like to express my gratitude to Dr Hao Zhang, Miss Xinxin Hou and Miss Chen Liang and all those who have helped me during this research.References
- R. Weissleder and M. J. Pittet, Imaging in the era of molecular oncolocy, Nature, 2008, 452, 580–589 CrossRef CAS PubMed.
- J. Geng, Z. Zhu, W. Qin, L. Ma, Y. Hu, G. G. Gurzadyan, B. Z. Tang and B. Liu, Near-infrared fluorescence amplified organic nanoparticles with aggregation-induced emission characteristics for in vivo imaging, Nanoscale, 2014, 6, 939–945 RSC.
- R. G. Aswathy, Y. Yoshida, T. Maekawa and D. S. Kumar, Near-infrared quantum dots for deep tissue imaging, Anal. Bioanal. Chem., 2010, 397, 1417–1435 CrossRef CAS PubMed.
- X. Sun, X. Huang, J. Guo, W. Zhu, Y. Ding, G. Niu, A. Wang, D. O. Kiesewetter, Z. L. Wang, S. Sun and X. Chen, Self-illuminating 64 Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging, J. Am. Chem. Soc., 2014, 136, 1706–1709 CrossRef CAS PubMed.
- H. Hu, P. Huang, O. J. Weiss, X. Yan, X. Yue, M. G. Zhang, Y. Tang, L. Nie, Y. Ma, G. Niu, K. Wu and X. Chen, PET and NIR optical imaging using self-illuminating (64) Cu-doped chelator-free gold nanoclusters, Biomaterials, 2014, 35, 9868–9876 CrossRef CAS PubMed.
- J. V. Frangioni, Self-illuminating quantum dots light the way, Nat. Biotechnol., 2006, 24, 326–328 CrossRef CAS PubMed.
- M. K. So, C. Xu, A. M. Loening, S. S. Gambhir and J. Rao, Self-illuminating quantum dot conjugates for in vivo imaging, Nat. Biotechnol., 2006, 24, 339–343 CrossRef CAS PubMed.
- Y. Xing, M. K. So, A. L. Koh, R. Sinclair and J. Rao, Improved QD-BRET conjugates for detection and imaging, Biochem. Biophys. Res. Commun., 2008, 372, 388–394 CrossRef CAS PubMed.
- Q. Wu and M. Chu, Self-illuminating quantum dots for highly sensitive in vivo real-time luminescent mapping of sentinel lymph nodes, Int. J. Nanomed., 2012, 7, 3433–3443 CAS.
- G. A. Quiñones, S. C. Miller, S. Bhattacharyya, D. Sobek and J. P. Stephan, Ultrasensitive detection of cellular protein interactions using bioluminescence resonance energy transfer quantum dot-based nanoprobes, J. Cell. Biochem., 2012, 113, 2397–2405 CrossRef PubMed.
- Y. Xing and J. Rao, Quantum dot bioconjugates for in vitro diagnostics & in vivo imaging, Cancer Biomarkers, 2008, 4, 307–319 CAS.
- M. K. So, A. M. Loening, S. S. Gambhir and J. Rao, Creating self-illuminating quantum dot conjugates, Nat. Protoc., 2006, 1, 1160–1164 CrossRef CAS PubMed.
- K. Oneill, S. K. Lyons, W. M. Gallagher, K. M. Curran and A. T. Byrne, Bioluminescent imaging: a critical tool in pre-clinical oncology research, J. Pathol., 2010, 220, 317–327 CAS.
- J. Dragavon, S. Blazquez, A. Rekiki, C. Samson, I. Theodorou, K. L. Rogers, R. Tournebize and S. L. Shorte, In vivo excitation of nanoparticles using luminescent bacteria, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 8890–8895 CrossRef CAS PubMed.
- K. I. Kim, J. H. Park, Y. J. Lee, T. S. Lee, J. J. Park, I. Song, S. S. Nahm, G. J. Cheon, S. M. Lim, J. K. Chung and J. H. Kang, In vivo bioluminescent imaging of α-fetoprotein-producing hepatocellular carcinoma in the diethylnitrosamine-treated mouse using recombinant adenoviral vector, J. Gene Med., 2012, 14, 513–520 CrossRef CAS PubMed.
- X. Xie, L. Li, X. Xiao, J. Guo, Y. Kong, M. Wu, W. Liu, G. Gao, J. L. Hsu, W. Wei, M. C. Hung and X. Xie, Targeted expression of BikDD eliminates breast cancer with virtually no toxicity in noninvasive imaging models, Mol. Cancer Ther., 2012, 11, 1915–1924 CrossRef CAS PubMed.
- Y. H. Lai, C. C. Lin, S. H. Chen and C. K. Tai, Tumor-specific suicide gene therapy for hepatocellular carcinoma by transcriptionally targeted retroviral replicating vectors, Gene Ther., 2015, 22, 155–162 CrossRef PubMed.
- H. A. Kim, K. Nam, M. Lee and S. W. Kim, Hypoxia/hepatoma dual specific suicide gene expression plasmid delivery using bio-reducible polymer for hepatocellular carcinoma therapy, J. Controlled Release, 2013, 171, 1–10 CrossRef CAS PubMed.
- Y. F. Peng, Y. H. Shi, Z. B. Ding, J. Zhou, S. J. Qiu and B. Hui, α-Fetoprotein promoter-driven Cre/LoxP-switched RNA interference for hepatocellular carcinoma tissue-specific target therapy, PLoS One, 2013, 8, e53072 CAS.
- C. Yuan, Y. An, J. Zhang, H. Li, H. Zhang, L. Wang and D. Zhang, Magnetic nanoparticles for targeted therapeutic gene delivery and magnetic-inducing heating on hepatoma, Nanotechnology, 2014, 25, 345101 CrossRef PubMed.
- J. H. Park, K. I. Kim, Y. J. Lee, T. S. Lee, K. M. Kim, S. S. Nahm, Y. S. Park, G. J. Cheon, S. M. Lim and J. H. Kang, Non-invasive monitoring of hepatocellular carcinoma in transgenic mouse with bioluminescent imaging, Cancer Lett., 2011, 310, 53–60 CrossRef CAS PubMed.
- W. B. Tan, S. Jiang and Y. Zhang, Quantum-dot based nanoparticles for targeted silencing of HRE2/neu gene via RNA interference, Biomaterials, 2007, 28, 1565–1571 CrossRef CAS PubMed.
- Y. Luo and Q. Wang, Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery, Int. J. Biol. Macromol., 2014, 64, 353–367 CrossRef CAS PubMed.
- M. D. Buschmann, A. Merzouki, M. Lavertu, M. Thibault, M. Jean and V. Darras, Chitosans for delivery of nucleic acids, Adv. Drug Delivery Rev., 2013, 65, 1234–1270 CrossRef CAS PubMed.
- R. Raftery, F. J. O’Brien and S. A. Cryan, Chitosan for gene delivery and orthopedic tissue engineering application, Molecules, 2013, 18, 5611–5647 CrossRef CAS PubMed.
- M. Hasegawa, Y. Tsukasaki, T. Ohyanagi and T. Jin, Bioluminescence resonance energy transfer coupled near-infrared quantum dots using GST-tagged luciferase for in vivo imaging, Chem. Commun., 2013, 49, 228–230 RSC.
- R. Alam, D. M. Fontaine, B. R. Branchini and M. M. Maye, Designing quantum rods for optimized energy transfer with firefly luciferase enzymes, Nano Lett., 2012, 12, 3251–3256 CrossRef CAS PubMed.
- C. Wu, K. Kawasaki, S. Ohgiya and Y. Ohmiya, Chemical studies on the BRET system between the bioluminescence of Cypridina and quantum dots, Photochem. Photobiol. Sci., 2011, 10, 1531–1534 CAS.
- J. Brogan, F. Li, W. Li, Z. He, Q. Huang and C. Y. Li, Imaging molecular pathways: reporter genes, Radiat. Res., 2012, 177, 508–513 CrossRef CAS PubMed.
- A. Ido, H. Uto, A. Moriuchi, K. Nagata, Y. Onaga, M. Onaga, T. Hori, S. Hirono, K. Hayashi, T. Tamaoki and H. Tsubouchi, Gene therapy targeting for hepatocellular carcinoma: selective and enhanced suicide gene expression regulated by a hypoxia-inducible enhancer linked to a human alpha-fetoprotein promoter, Cancer Res., 2001, 61, 3016–3021 CAS.
- M. Iyer, L. Wu, M. Carey, Y. Wang, A. Smallwood and S. S. Gambhir, Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 14595–14600 CrossRef CAS PubMed.
- M. L. Schipper, A. Weber, M. Béhé, R. Göke, W. Joba, H. Schmidt, T. Bert, B. Simon, R. Arnold, A. E. Heufelder and T. M. Behr, Radioiodine treatment after sodium iodide symporter gene transfer is a highly effective therapy in neuroendocrine tumor cells, Cancer Res., 2003, 63, 1333–1338 CAS.
- M. Han, X. Gao, J. Z. Su and S. Nie, Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules, Nat. Biotechnol., 2001, 19, 631–635 CrossRef CAS PubMed.
Footnote |
| † The author contributed equally to this work and should be considered as co-first author. |
| This journal is © The Royal Society of Chemistry 2015 |