Polydopamine coated SPEEK membrane for a vanadium redox flow battery
Jingyu Xi*a,
Wenjing Daia and
Lihong Yu*b
aInstitute of Green Chemistry and Energy, Graduate School at Shenzhen, Tsinghua University, Shenzhen 518055, China. E-mail: xijy@tsinghua.edu.cn
bSchool of Applied Chemistry and Biological Technology, Shenzhen Polytechnic, Shenzhen 518055, China. E-mail: yulihong@szpt.edu.cn
First published on 1st April 2015
Abstract
In this work, sulfonated poly(ether ether ketone) (SPEEK) membranes have been first modified by a polydopamine (PDA) coating and then investigated as an ion exchange membrane for a vanadium redox flow battery (VRFB). The SEM results showed that the PDA film was successfully coated on the surface of the SPEEK membrane. The PDA film can serve as a blocking layer to protect the substrate SPEEK membrane from being corroded by the highly oxidative vanadium electrolyte. The obtained PDA coated SPEEK membranes showed much lower vanadium ion permeability, accompanied by higher mechanical strength and thermal stability than the pristine SPEEK membrane. Consequently, the VRFB with the optimal PDA/SPEEK membrane exhibited much higher coulombic efficiency and better discharge capacity retention than the pristine SPEEK membrane. This indicates that the PDA coated SPEEK membranes are of significant interest for VRFB applications.
1. Introduction
Dwindling fossil energy and increasing energy demands have forced us to develop power systems using renewable energy, such as solar and wind. A power system using renewable energy should be combined with an energy storage system due to its intermittent nature and low energy density. Various redox flow batteries (RFB) have been developed to store energy on the medium to large scale, particularly in applications such as load levelling, power quality control and facilitating renewable energy deployment. In particular, the vanadium redox flow battery (VRFB) has been considered as a promising technology for large-scale energy storage in the future due to its salient advantages such as long lifespan, fast response time and flexible design.1–10In a VRFB, the ion exchange membrane (IEM) is a critical material that separates the positive and negative electrolytes while transporting ions. At present, the most widespread adopted IEMs in commercialized VRFB are Nafion series membranes (Dupont) due to its high chemical stability and proton conductivity. Despite these advantages, Nafion series membranes face serious limiting factors for the further commercialization of VRFB, especially with regard to the high cost and high vanadium ion permeability issues. Massive efforts have been engaged in various modifications of Nafion membranes, but they cannot resolve the substantive issue of the high cost.11–16 Partially fluorinated membranes (PTFE, PVDF etc.) and non-fluorinated membranes like sulfonated poly(arylene thioether ketone) (SPTK) and sulfonated poly(arylene thioether ketone ketone) (SPTKK) were also developed for application in VRFB.17–19 It is known that developing low-cost, long-life, and high-selectivity ion exchange membrane material is one of the most important prerequisites for promoting the large-scale application of VRFB.20,21 To date, hydrocarbon skeleton structure based ion exchange membrane, especially sulfonated poly(ether ether ketone) (SPEEK), is considered to be the most attractive candidate to replace Nafion series membrane due to its lower production cost, higher ion selectivity as well as easier scale preparation.20–23 Some modification methods, such as polymer blend,24 organic–inorganic hybrid,25–27 and inert porous substrate pore-filling,27,28 can significantly improve the mechanical strength and chemical stability of the SPEEK membrane, making it more suitable to apply in long-life VRFB system.
Dopamine molecule contains catechol and amine functional groups (see Fig. 1). In the presence of oxygen, the dopamine monomer can be easily self-polymerized and oxygenated in the dopamine solution (pH = 8.5), forming a thin adherent polydopamine (PDA) layer on the surface of a wide range of inorganic and organic solid materials, such as metal, metal oxides and polymer.29–34 PDA is characterized by extraordinary adhesion properties providing efficient and universal surface coating for diverse settings. It has been established that applying a PDA coating is a quite simple and environmental friendly surface modification method which has been widely applied in the fields of energy, environment, medicine, etc.35–42 In this paper, PDA coating was adopted for the surface modification of SPEEK membrane. The PDA layer can serve as a blocking layer to prevent the crossover of vanadium ions and protect the substrate SPEEK membrane from being corroded by the highly oxidative vanadium electrolyte as well. The properties of the resultant PDA coated SPEEK membranes are evaluated via SEM, the proton conductivity, vanadium ion permeability, mechanical strength, thermal stability and VRFB cell performance. And substantially all of the tested properties are significantly enhanced compared to the pristine SPEEK membrane. Thus the optimal PDA-coated SPEEK membrane exhibits much higher coulombic efficiency and better charge capacity retention than the pristine SPEEK membrane and Nafion 117 membrane when used in VRFB system, which implies that the PDA coated SPEEK membranes hold considerable promise in low-cost, high-efficient and long-life VRFB systems.
2. Experimental
2.1 Materials
Poly(ether ether ketone) (PEEK) (Victrex, PEEK 450 G) was pretreated by washing and then drying at 80 °C for 24 h in vacuum. Dopamine hydrochloride was purchased from Sigma Aldrich (Australia). All the other analytical reagents, including 1.5 M Tris–HCl buffer solution, N,N-dimethylformamide (DMF), H2SO4, NaCl, NaOH, MgSO4·7H2O and VOSO4·4H2O, were purchased from local chemical suppliers and used without further purification. Nafion 117 membrane was purchased from DuPont Company.2.2 Membrane preparation
SPEEK membrane was prepared by solvent casting method according to our previous report.25,26 The degree of sulfonation (DS) of the SPEEK membrane used in this work is 0.70. The thickness of the wet SPEEK membrane is 70 μm. As shown in Fig. 1, the PDA coated membranes were prepared by a simple immersion process of completely dipping SPEEK membrane into the 1.5 g L−1 of the dopamine aqueous solution (pH = 8.5) for different time at room temperature and then washing with deionized water to remove the residual dopamine and dark brown polydopamine particles. After that, the resulting membranes were dried at 40 °C for 6 h. The PDA coated SPEEK membranes were denoted as PDA/SPEEK x h, in which x means the dipping hours in the dopamine solution.2.3 Membrane characterization
To measure the VO2+ permeability, the membrane was exposed to a solution of 1.5 mol L−1 VOSO4 in 3 mol L−1 H2SO4 (40 mL in the left reservoir) and a solution of 1.5 mol L−1 MgSO4 in 3 mol L−1 H2SO4 (40 mL in the right reservoir). The two solutions were continuously magnetically stirred to avoid the concentration polarization. The effective area of the membrane was 7 cm2. The concentration of VO2+ in the right reservoir was monitored as a function of time using a Spectrum lab 752S UV-vis spectrophotometer (China, Leng Guang Tech.). The ion selectivity of membrane is defined as the ratio of the proton conductivity and the VO2+ permeability.
The TGA (Thermal Gravity Analysis) curves and DTA (Differential Thermal Analysis) curves of the membranes were obtained on a thermal gravimetric analyzer (Perkin Elmer Pyris 1 TGA), operating from 100 °C to 750 °C at a rate of 10 °C min−1 in N2 gas atmosphere.
2.4 VRFB single cell performance
The VRFB single cell was manufactured by sandwiching a membrane (70 mm × 70 mm) between two heat activated graphite felt electrodes (50 mm × 50 mm × 5 mm), with two graphite polar plates as current collectors.24,27 The starting negative and positive electrolytes were two 50 mL of 2.0 mol L−1 VO2+/V3+ (mol/mol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 2.0 mol L−1 H2SO4 solutions, and they were cycled using peristaltic pumps at a flow rate of 60 mL min−1. The cell was charged and discharged using Neware CT-3008W-5V6A battery testing system. The VRFB single cell was firstly charged to 50% state of charge (SOC) at the current density of 60 mA cm−2. Then the self-discharge test immediately began and stopped until the single cell open circuit voltage (OCV) was below 0.8 V. The cycle life test was carried out at the constant current density of 80 mA cm−2 with the cut-off voltages setting at 1.65 V and 0.8 V, respectively.
1) in 2.0 mol L−1 H2SO4 solutions, and they were cycled using peristaltic pumps at a flow rate of 60 mL min−1. The cell was charged and discharged using Neware CT-3008W-5V6A battery testing system. The VRFB single cell was firstly charged to 50% state of charge (SOC) at the current density of 60 mA cm−2. Then the self-discharge test immediately began and stopped until the single cell open circuit voltage (OCV) was below 0.8 V. The cycle life test was carried out at the constant current density of 80 mA cm−2 with the cut-off voltages setting at 1.65 V and 0.8 V, respectively.
3. Results and discussion
3.1 Membrane preparation and morphology
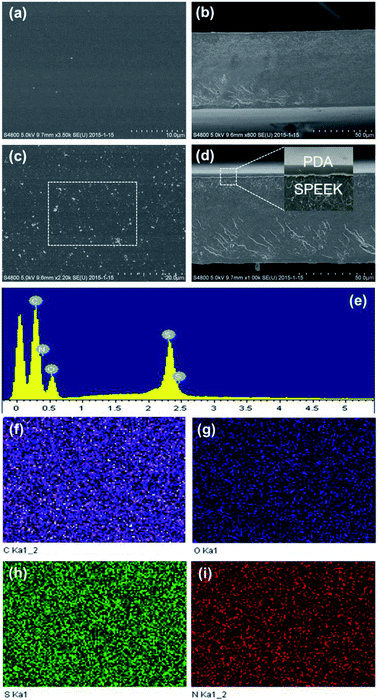
As described in Fig. 1(a)–(c), polydopamine-coated SPEEK membranes are prepared by simply dipping the dried neat SPEEK membrane in the prepared aqueous-based dopamine solution for different time. The obtained SPEEK membrane appears light yellow, homogeneous and transparent, as shown in Fig. 1(a). After PDA coating, the resultant PDA/SPEEK membrane turns gradually from light yellow to deep yellow with the dipping time as shown in Fig. 1(c)–(f). The obvious color change indicates the surface coating of SPEEK membrane by PDA. The detailed description of polymerization mechanism of dopamine is shown in Fig. 1(g).43 Beginning with the self-oxidization of dopamine to yield dopaquinone, a series of complex intra- and inter-molecular reactions occur that finally results in PDA.To probe the morphology of the as-prepared membranes, their SEM images are obtained and shown in Fig. 2. Notable changes of surface and cross-section morphology were observed after PDA coating. It can be observed from Fig. 2(a) and (c) that the surface of SPEEK membrane changes from bare to bubble-like, which is formed by DA polymerization. Fig. 2(b) and (d) show the cross-section SEM images of SPEEK and PDA/SPEEK 0.5 h membranes. It can be easily found that the PDA layer is visible, and polymerized well on the surface of SPEEK membrane. Moreover, the region encircled by white dash line in Fig. 2(c) is investigated by EDX, and the EDX element mapping of PDA/SPEEK 0.5 h membrane is illustrated in Fig. 2(e)–(i). The element S (green color in Fig. 2(h)) representing SPEEK and element N (red color in Fig. 2(i)) representing PDA were uniformly dispersed, indicating the homogeneous morphology of the composite membranes. All the SEM results confirm that the PDA film is successfully coated on the surface of SPEEK membrane.
3.2 Mechanical properties and thermal analysis
As the key element in VRFB, IEMs must have excellent mechanical and thermal stabilities for long-time operation. The mechanical properties of Nafion 117, SPEEK and PDA/SPEEK membranes with different PDA dipping time are summarized in Table 1. The breaking strength of the PDA/SPEEK membranes increases with the PDA dipping time. The benchmark-Nafion 117 has the lowest breaking strength (21.4 MPa). The breaking strength increases from 30.6 MPa for bare SPEEK membrane to 40.1 MPa for PDA/SPEEK 0.5 h membrane; and the breaking strength of the PDA/SPEEK membranes increases slightly as dipping time prolongs. The percentage elongation of the SPEEK and PDA/SPEEK membranes is between 120–150%, which is much smaller than that of Nafion 117 (218%). It is obvious that PDA coating is an effective reinforcement for SPEEK membrane owing to the strong hydrogen bonds interaction between PDA and SPEEK.| Samples | Proton conductivity (mS cm−1) | VO2+ permeability (10−7 cm2 min−1) | Selectivity (104 S min cm−3) | Breaking strength (MPa) | Percentage elongation (%) |
|---|---|---|---|---|---|
| Nafion 117 | 31.89 | 34.90 | 0.91 | 21.4 | 218 |
| SPEEK | 16.53 | 10.44 | 1.58 | 30.6 | 150 |
| PDA/SPEEK 0.5 h | 12.52 | 1.67 | 7.48 | 40.1 | 128 |
| PDA/SPEEK 1.0 h | 4.69 | 0.70 | 6.66 | 44.9 | 134 |
| PDA/SPEEK 2.0 h | — | 0.69 | — | 44.4 | 121 |
| PDA/SPEEK 3.0 h | — | 0.56 | — | 48.1 | 128 |
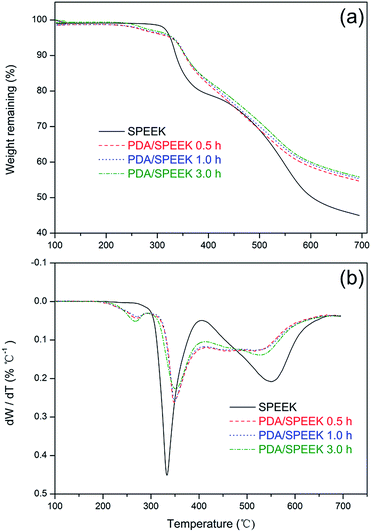
The thermal properties of the as-prepared membranes are determined by TGA and DTA, and the results are presented in Fig. 3. Consistent with our previous study,25–27 the thermal decomposition of SPEEK occurs in two stages, while all the PDA/SPEEK membranes undergo three weight-loss stages, which can also be reflected by the number of peaks in the DTA curve in separate temperature ranges. For PDA/SPEEK membranes, the stage (250–280 °C) is probably assigned to the heating decomposition of tiny amounts PDA particles at outermost layer. For all tested membranes, the stage (290–350 °C) is attributed to the degradation of sulfonic acid group of SPEEK; the stage (400–550 °C) is assigned to the decomposition of the SPEEK backbone. It can be seen that as PDA dipping time prolongs, the TGA curves shift slightly toward higher temperatures and the remanent weight ratio of sample membranes increases. The degradation of sulfonic acid group for PDA/SPEEK membranes is notably increased than that for pristine SPEEK. For pristine SPEEK, the sulfonic acid groups degradate at 339 °C, and for PDA/SPEEK 0.5 h membrane the sulfonic acid groups degradate at 348 °C. And the remanent weight ratio of SPEEK membrane is 44.9% while the remanent weight ratio of PDA/SPEEK 0.5 h membrane is 54.8%. From all the above results, we could actually conclude that the PDA/SPEEK membranes exhibit a higher thermal stability of compared to pure SPEEK membrane due to the protection of PDA. All the results are similar to the values as elsewhere reported.44
3.3 Proton conductivity, permeability of VO2+ and ion selectivity
The data of the proton conductivity, VO2+ permeability and selectivity of all membranes are summarized in Table 1. As shown in Table 1, the pristine SPEEK membrane acquires a proton conductivity of 16.53 mS cm−1. It's not very satisfactory that PDA coating results in the decrease of the proton conductivity of the pristine SPEEK membrane. The longer the dipping time, the more degradation the proton conductivity of the pristine SPEEK membrane exhibits. When the dipping time is above 2.0 h, the proton conductivity of the tested membrane is beyond the scope of the instrument. This phenomenon can be explained as follows: more PDA coats on the surface of SPEEK membrane as dipping time prolongs, which leads to the dominance of the blocking effect on proton transport for the formed PDA film.45 Nevertheless, the proton conductivity of the PDA/SPEEK 0.5 h membrane (12.52 mS cm−1) is acceptable and can fully meet the VRFB testing requirements. Moreover it illustrates excellent VRFB cell performance despite the lower proton conductivity compared to Nafion 117 membrane (31.89 mS cm−1), as verified by the following results.From Table 1, we can see that the VO2+ permeability of SPEEK membrane is much lower than that of Nafion 117 membrane (10.44 × 10−7 cm2 min−1 vs. 34.90 × 10−7 cm2 min−1), due to their different microstructures-the degree of hydrophobic/hydrophilic separation.23 Fig. 4 displays the VO2+ concentration as a function of time under identical conditions for different membranes. The VO2+ concentration increases with time for the pristine SPEEK membrane, which is nearly comparable to that for Nafion 117 membrane. As expected, the concentration for PDA/SPEEK membranes becomes much lower than that for pristine SPEEK membrane. It is also found that the VO2+ permeability across the PDA/SPEEK membranes decreases with the coating time and remains nearly constant when the coating time exceeds 1.0 h, which can be also seen from Table 1. Given that PDA coating layer can not only effectively block the vanadium ion permeability, but also simultaneously increase the membrane resistance, namely improving the coulombic efficiency (CE) of VRFB while reducing the voltage efficiency (VE), therefore we define a comprehensive factor-selectivity to evaluate membrane performance, which is defined as the ratio of proton conductivity and vanadium ion permeability. Generally a higher selectivity value reflects better VRFB performance.23 From Table 1, the PDA/SPEEK 0.5 h membrane shows the highest selectivity of 7.48 × 104 S min cm−3, which far exceeds that of Nafion 117 membrane (0.91 × 104 S min cm−3). Therefore the PDA/SPEEK 0.5 h membrane is selected for subsequent VRFB performance tests.
 | ||
| Fig. 4 Time-dependent concentration changes of VO2+ in MgSO4 solution across the SPEEK and PDA/SPEEK membranes. | ||
3.4 VRFB single cell performance
The self-discharge curves of VRFBs with Nafion 117, SPEEK, and PDA/SPEEK 0.5 h membranes are compared in Fig. 5. The open circuit voltage (OCV) gradually decreased with time at first and then sharply dropped to 0.8 V. The time to reach 0.8 V was 46, 64, and 216 h for Nafion, SPEEK, and PDA/SPEEK 0.5 h membranes, respectively. The OCV for PDA/SPEEK 0.5 h membrane decays much more slowly than that for SPEEK and Nafion 117, which is in good accordance with the result of the VO2+ permeability test.Fig. 6 illustrates the cell efficiency of VRFBs with Nafion 117, SPEEK, and PDA/SPEEK 0.5 h membranes at constant current densities from 40 to 200 mA cm−2. The CE of VRFBs with Nafion and SPEEK increases with current density while the CE of VRFB with PDA/SPEEK 0.5 h membrane is much higher (∼98.5%) and maintains the high level at all current densities, coinciding with the results of Fig. 4 and 5. The energy efficiency (EE) of VRFBs with all three membranes decreases with current density while the VRFBs with Nafion membrane exhibits the lowest EE at all current densities. The EE of VRFB with PDA/SPEEK 0.5 h membrane is higher than that of pristine SPEEK at 40–80 mA cm−2. However, the EE of VRFB with SPEEK membrane becomes slightly higher than that of PDA/SPEEK 0.5 h at 100–200 mA cm−2, which can be ascribed to the relatively higher resistance of PDA/SPEEK 0.5 h membrane after PDA coating. However, it is no doubt that PDA/SPEEK membrane has very obvious advantage in the cell efficiency at low current densities.
 | ||
| Fig. 6 Coulombic efficiency (CE) and energy efficiency (EE) of VRFBs with Nafion 117, SPEEK, and PDA/SPEEK 0.5 h membranes at constant current densities from 40 to 200 mA cm−2. | ||
To further investigate the stability of the membranes in actual VRFB operation, the VRFBs assembled with three different membranes were run continuously for 150 charge–discharge cycles at 80 mA cm−2. It can be seen from Fig. 7(a) that the CEs of VRFBs with Nafion 117, SPEEK, and PDA/SPEEK 0.5 h membranes all show no obvious decline during 150 cycles with a rank of PDA/SPEEK 0.5 h > SPEEK > Nafion. As shown in Fig. 7(b), the EE of the VRFBs based on both SPEEK and PDA/SPEEK 0.5 h membranes remain constant and are almost the same during all 150 cycles, higher than that of Nafion 117. The cycle performance of CE and EE agrees well with the efficiency result in Fig. 7. This indicates that the tested membranes could survive the severe VRFB operation conditions. After 150 cycles, the discharge capacity retention of VRFBs with Nafion, SPEEK, and PDA/SPEEK 0.5 h membranes are 34%, 19% and 70%, respectively, as shown in Fig. 7(c). Despite the good chemical stability, Nafion membrane suffers high vanadium ion permeability, which results poor discharge capacity retention of VRFB. For SPEEK membrane, it has lower vanadium ion permeability compared with Nafion membrane. However, it is not very stable due to its swelling effect in strong oxidizing vanadium solutions, and consequently the discharge capacity of VRFB with SPEEK decays slowly at first, but then the attenuation is accelerated. On the contrary, the PDA/SPEEK 0.5 h membrane has the lowest vanadium ion permeability among the three membranes as shown in Fig. 4, and with the further protection of PDA film, it possesses a superior chemical stability in the strong oxidizing and acidic vanadium solutions. The discharge capacity retention of VRFB with PDA/SPEEK 0.5 h is the highest (70%) among the three membranes, which suggests that PDA/SPEEK membranes can greatly promote the development of IEMs for the long-life VRFB application.
 | ||
| Fig. 7 Cycle performance of VRFBs with Nafion 117, SPEEK, and PDA/SPEEK 0.5 h membranes: (a) coulombic efficiency; (b) energy efficiency; (c) discharge capacity retention. | ||
4. Conclusions
Polydopamine coated sulfonated poly(ether ether ketone) membranes (PDA/SPEEK) are successfully prepared and first applied in VRFB system. The PDA layer can effectively block the transport of vanadium ions and simultaneously protect the substrate SPEEK membrane from corrosion caused by the vanadium electrolyte. Therefore, a much higher coulombic efficiency and better discharge capacity retention are obtained for VRFB with the preferred membrane (PDA/SPEEK 0.5 h membrane) as compared with the benchmark-Nafion 117 membrane. Based on all the results above, the low-cost PDA coated SPEEK membranes have great potential for usage in highly efficient and long-life VRFB systems.Acknowledgements
This work was supported by the Knowledge Innovation Program of Shenzhen (JCYJ20130402145002403) and National Natural Science Foundation of China (20973099).Notes and references
- B. Dunn, H. Kamath and J. Tarascon, Science, 2011, 334, 928 CrossRef CAS PubMed.
- Z. Yang, J. Zhang, M. C. W. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 357 CrossRef PubMed.
- W. Wang, Q. Luo, B. Li, X. Wei, L. Li and Z. Yang, Adv. Funct. Mater., 2013, 23, 970 CrossRef CAS.
- M. Skyllas-Kazacos, M. H. Chakrabarti, S. A. Hajimolana, F. S. Mjalli and M. Saleem, J. Electrochem. Soc., 2011, 158, R55 CrossRef CAS PubMed.
- A. Parasuraman, T. M. Lim, C. Menictas and M. Skyllas-Kazacos, Electrochim. Acta, 2013, 101, 27 CrossRef CAS PubMed.
- C. Ding, H. Zhang, X. Li, T. Liu and F. Xing, J. Phys. Chem. Lett., 2013, 4, 128 Search PubMed.
- B. R. Chalamala, T. Soundappan, G. R. Fisher, M. R. Anstey, V. V. Viswanathan and M. L. Perry, Proc. IEEE, 2014, 102, 976 CrossRef CAS.
- P. Leung, X. Li, C. P. Leon, L. Berlouis, C. T. J. Lowa and F. C. Walshab, RSC Adv., 2012, 2, 10125 RSC.
- W. Zhang, J. Xi, Z. Li, H. Zhou, L. Liu, Z. Wu and X. Qiu, Electrochim. Acta, 2013, 89, 429 CrossRef CAS PubMed.
- A. Tang, J. Bao and M. Skyllas-Kazacos, J. Power Sources, 2011, 196, 10737 CrossRef CAS PubMed.
- J. Xi, Z. Wu, X. Teng, Y. Zhao, L. Chen and X. Qiu, J. Mater. Chem., 2008, 18, 1232 RSC.
- J. Zeng, C. Jiang, Y. Wang, J. Chen, S. Zhu, B. Zhao and R. Wang, Electrochem. Commun., 2008, 10, 372 CrossRef CAS PubMed.
- J. Xi, Z. Wu, X. Qiu and L. Chen, J. Power Sources, 2007, 166, 531 CrossRef CAS PubMed.
- N. Wang, S. Peng, D. Lu, S. Liu, Y. Liu and K. Huang, J. Solid State Electrochem., 2012, 4, 1577 CrossRef.
- X. Teng, Y. Zhao, J. Xi, Z. Wu, X. Qiu and L. Chen, J. Membr. Sci., 2009, 341, 149 CrossRef CAS PubMed.
- S. Lu, C. Wu, D. Liang, Q. Tanab and Y. Xiang, RSC Adv., 2014, 4, 24831 RSC.
- X. Luo, Z. Lu, J. Xi, Z. Wu, W. Zhu, L. Chen and X. Qiu, J. Phys. Chem. B, 2005, 109, 20310 CrossRef CAS PubMed.
- W. Wei, H. Zhang, X. Li, Z. Mai and H. Zhang, J. Power Sources, 2012, 208, 421 CrossRef CAS PubMed.
- D. Y. Chen, S. J. Wang, M. Xiao and Y. Z. Meng, Energy Environ. Sci., 2010, 3, 622 CAS.
- X. Li, H. Zhang, Z. Mai, H. Zhang and I. Vankelecom, Energy Environ. Sci., 2011, 4, 1147 CAS.
- H. Prifti, A. Parasuraman, S. Winardi, T. M. Lim and M. Skyllas-Kazacos, Membranes, 2012, 2, 275 CrossRef CAS PubMed.
- J. Xi, Z. Li, L. Yu, B. Yin, L. Wang, L. Liu, X. Qiu and L. Chen, J. Power Sources, 2015, 285, 195 CrossRef CAS PubMed.
- S. Winardi, S. C. Raghu, M. O. Oo, Q. Yan, N. Wai, T. M. Lim and M. Skyllas-Kazacos, J. Membr. Sci., 2014, 450, 313 CrossRef CAS PubMed.
- Z. Li, W. Dai, L. Yu, L. Liu, J. Xi, X. Qiu and L. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 18885 CAS.
- Z. Li, W. Dai, L. Yu, J. Xi, X. Qiu and L. Chen, J. Power Sources, 2014, 257, 221 CrossRef CAS PubMed.
- W. Dai, L. Yu, Z. Li, J. Yan, L. Liu, J. Xi and X. Qiu, Electrochim. Acta, 2014, 132, 200 CrossRef CAS PubMed.
- W. Dai, Y. Shen, Z. Li, L. Yu, J. Xi and X. Qiu, J. Mater. Chem. A, 2014, 2, 12423 CAS.
- W. Wei, H. Zhang, X. Li, Z. Mai and H. Zhang, J. Power Sources, 2012, 208, 421 CrossRef CAS PubMed.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057 CrossRef CAS PubMed.
- H. Lee, S. Dellatore, W. Miller and P. Messersmith, Science, 2007, 318, 426 CrossRef CAS PubMed.
- L. Yang, S. Phua, J. Teo, C. Toh, S. Lau, J. Ma and X. Lu, ACS Appl. Mater. Interfaces, 2011, 3, 3026 CAS.
- S. Hwang, D. Kang, R. Ruoff, H. Shin and Y. Par, ACS Nano, 2014, 7, 6739 CrossRef PubMed.
- B. Yu, J. Liu, S. Liu and F. Zhou, Chem. Commun., 2010, 46, 5900 RSC.
- J. Waite, Nat. Mater., 2008, 7, 8 CrossRef CAS PubMed.
- H. Lee, J. Rho and P. Messersmith, Adv. Mater., 2009, 21, 431 CrossRef CAS PubMed.
- H. Ham, Z. Liu, K. Lau, H. Lee and P. Messersmith, Angew. Chem., Int. Ed., 2011, 50, 732 CrossRef CAS PubMed.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244 RSC.
- S. Hong, K. Kim, H. Wook, S. Park, K. Lee, D. Lee and H. Lee, Nanomedicine, 2011, 6, 793 CrossRef CAS PubMed.
- S. Ku, J. Ryu, S. Hong, H. Lee and C. Park, Biomaterials, 2010, 31, 2535 CrossRef CAS PubMed.
- J. Kuang and P. Messersmith, Langmuir, 2012, 28, 7258 CrossRef CAS PubMed.
- Y. Lee, M. Ryou, M. Seo, J. Choi and Y. Lee, Electrochim. Acta, 2013, 113, 433 CrossRef CAS PubMed.
- M. d'Ischia, A. Napolitano, V. Ball, C. Chen and M. Buehler, Acc. Chem. Res., 2014, 47, 3541 CrossRef PubMed.
- J. Chen, X. Chen, X. Yin, J. Ma and Z. Jiang, J. Membr. Sci., 2009, 344, 136 CrossRef CAS PubMed.
- Y. He, J. Wang, H. Zhang, T. Zhang, B. Zhang, S. Cao and J. Liu, J. Mater. Chem. A, 2014, 2, 9548 CAS.
- K. Kim, E. Yang, M. Lee, K. Chae, C. Kim and I. Kim, Water Res., 2014, 54, 62 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |