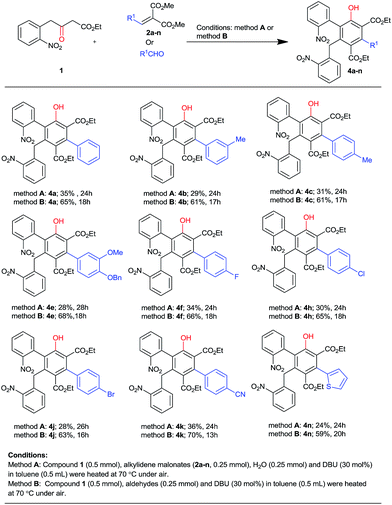
Organocatalysed Michael addition on arylmethylidenemalonates involving 4-(2-nitrophenyl)acetoacetate: diversity-oriented access to 8,9-dihydropyrido[1,2-a]indol-6(7H)-one and salicylate scaffolds†
Anvita Srivastava,
Soumen Biswas,
Shivendra Singh,
Shaikh M. Mobin and
Sampak Samanta*
Indian Institute of Technology Indore, 452017, Indore, Madhya Pradesh, India. E-mail: sampaks@iiti.ac.in; sampak_s1@yahoo.com; Fax: +91-731-2364182; Tel: +91-731-2438742
First published on 6th March 2015
Abstract
Excellent diastereoselective (≤96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) preparation of new functionalized 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-ones has been achieved in 61–81% yields through a one-pot reductive cycloaromatization–lactamization sequence reaction of Michael adducts using Zn/NH4Cl as a reducing agent. In addition, one-pot synthesis of sterically demanding hexa-substituted salicylate derivatives has been successfully realized via a tandem reaction of alkylidene malonates or aldehydes with 2-nitrophenyl-β-keto ester under aerobic conditions using DBU as an organocatalyst.
4) preparation of new functionalized 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-ones has been achieved in 61–81% yields through a one-pot reductive cycloaromatization–lactamization sequence reaction of Michael adducts using Zn/NH4Cl as a reducing agent. In addition, one-pot synthesis of sterically demanding hexa-substituted salicylate derivatives has been successfully realized via a tandem reaction of alkylidene malonates or aldehydes with 2-nitrophenyl-β-keto ester under aerobic conditions using DBU as an organocatalyst.
The Michael addition plays a prominent role in C–X (X = C, O, N, S, P etc.) bond forming reactions in synthetic organic and medicinal chemistry.1 Among them, organocatalytic Michael additions of carbon-centered nucleophiles to electron deficient substituted alkenes have been recognized in the chemical community as one of the most flexible, atom-economical, straightforward and widely employed chemical transformations for the construction of C–C bonds with multiple stereocenters.2 In recent years, commercially available double activated olefins such as alkylidene malonates have been used as versatile Michael acceptors3,4 because these diester functionalities in turn can be useful for the preparation of biologically active lactone, lactam, imide etc.5 For instance, Barbas4a and other groups have independently developed enantioselective Michael additions of carbon-centered donors such as acyclic and cyclic ketones,4a–e 1,3-dicarbonyls, α,β-unsaturated γ-butyrolactams,4f nitroalkanes4g to alkylidene malonates using enamine or H-bonding based organocatalysts for promoting these reactions. Interestingly, in 2013, the Maruoka group6 has reported on the highly enantioselective cyanation of alkylidene malonates using phase transfer catalysis. Furthermore, simple base promoted non-asymmetric versions of conjugate additions of 2-cyanoacetamide, malononitrile and nitromethane to alkylidene have been reported by the Fan and Stephens groups, respectively.7 Even with these considerable progresses, the addition of β-keto esters to alkylidene malonate has never been addressed systematically in the literature. Therefore, we are interested in the development of organocatalytic efficient systems for the Michael addition reaction of simple functionalized β-keto esters with alkylidene malonate, leading to a series of possible synthetically valuable functional group-rich Michael addition products.
Nowadays, easily accessible 2-nitrobenzyl-substituted-β-keto ester derivatives are frequently employed as versatile reactive intermediates in synthetic organic chemistry because this 2-nitrobenzyl ketone moiety can be easily converted into the biologically as well as pharmaceutically important class of 2-substituted indole derivatives in a one-pot manner.8 Despite its potential applications, examples of the Michael reaction with this β-keto ester have been rare. Only one successful example was reported very recently by Bonjoch and co-workers9 towards the synthesis of morphan derivatives utilizing 4-(2-nitrophenyl) acetoacetate as a Michael donor in a reaction with β-substituted acrolein catalyzed by diphenylprolinol silyl ether. Against this background, we surmised that this β-keto ester may act as a potential nucleophile under the influence of a stronger Brønsted base and thus it would attack relatively poor Michael acceptors like alkylidene malonates to access the synthetically valuable Michael adduct (III) which is flanked by the 2-nitrobenzyl ketone moiety as a surrogate for a 2-substituted indole derivative (Scheme 1).
 | ||
| Scheme 1 Synthetic strategy for 2-substituted indole derivatives possessing tri-ester functionalities. | ||
As part of our continued research goal directed towards the use of small organic molecules as catalysts for the synthesis of important class of N/S/O-heterocyclic compounds through the Michael reaction as a key step,10 herein we report a simple, convenient, catalytic and efficient Michael addition reaction of ethyl 4-(2-nitrophenyl)acetoacetate with a variety of β-aryl-alkylidene malonates in the presence of DBU under heating conditions to provide high yields (up to 85%) of 3-ethoxycarbonyl-1,1-dimethoxycarbonyl-5-(2-nitrophenyl)-2-aryl-pentan-4-one derivatives as Michael adducts. Finally, the resulting Michael adducts have been elaborated to the previously unknown functionalized 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-ones in a highly diastereoselective manner.
At the beginning, the Michael addition reaction was carried out in toluene at 40 °C by choosing ethyl 4-(2-nitrophenyl)acetoacetate (1) and dimethyl 2-benzylidenemalonate (2a) as the model substrates in the presence of DBU (20 mol%). Interestingly, after 24 h, we isolated a non-separable diastereomeric mixture of Michael adduct 3a in a combined yield of 45% (entry 1, Table 1) and diastereomeric ratio 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. Upon increasing the reaction temperature to 60 °C, very good yield (71%, entry 2) of 3a was obtained along with a trace amount of unexpected hexa-substituted phenol 4a. By increasing the amount of catalyst loading (30 mol%), molar ratio of β-keto ester 1 (2 equiv.) and temperature to 70 °C, a lower yield of Michael adduct 3a was obtained due to the formation of unintended product 4a in considerable amounts (15–27%, entries 3 and 5). Moreover, the yield of 4a was further increased by adding one equivalent of water in toluene (entry 5). This is due to the instability of alkylidene product 2a under the reaction conditions, which may transform into benzaldehyde through a retro-Knoevenagel reaction4a as verified by our control experiments.11 It should be noted that a lower yield of phenol derivative 4a was observed under identical conditions when molecular sieves were used for this reaction (entry 4). Furthermore, replacement of toluene by other organic solvents such as EtOH, MeCN, DMF and THF led to inferior results (15–41% yields of 3a, entries 7–10). Next, we investigated several catalysts such as DABCO, Hünig’s base, L-proline, K2CO3, Cs2CO3, M(OTf)3 [M = Gd, Zn, In, Yb] in toluene under heating conditions (entries 11–19). In all these cases unacceptable results were obtained.
40. Upon increasing the reaction temperature to 60 °C, very good yield (71%, entry 2) of 3a was obtained along with a trace amount of unexpected hexa-substituted phenol 4a. By increasing the amount of catalyst loading (30 mol%), molar ratio of β-keto ester 1 (2 equiv.) and temperature to 70 °C, a lower yield of Michael adduct 3a was obtained due to the formation of unintended product 4a in considerable amounts (15–27%, entries 3 and 5). Moreover, the yield of 4a was further increased by adding one equivalent of water in toluene (entry 5). This is due to the instability of alkylidene product 2a under the reaction conditions, which may transform into benzaldehyde through a retro-Knoevenagel reaction4a as verified by our control experiments.11 It should be noted that a lower yield of phenol derivative 4a was observed under identical conditions when molecular sieves were used for this reaction (entry 4). Furthermore, replacement of toluene by other organic solvents such as EtOH, MeCN, DMF and THF led to inferior results (15–41% yields of 3a, entries 7–10). Next, we investigated several catalysts such as DABCO, Hünig’s base, L-proline, K2CO3, Cs2CO3, M(OTf)3 [M = Gd, Zn, In, Yb] in toluene under heating conditions (entries 11–19). In all these cases unacceptable results were obtained.
| Entry | Catalyst | Solvent | T °C | T/h | drb | Yieldc (%) 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a 4a |
|
|---|---|---|---|---|---|---|---|
| a Unless otherwise specified, all the reactions were performed with ethyl 4-(2-nitrophenyl)acetoacetate (1, 0.25 mmol), dimethyl 2-benzylidenemalonate (2a, 0.3 mmol) and catalyst (20 mol%) in the specified dry solvent (0.5 mL) and at the specified temperature under air.b Diastereomeric ratio was determined by the corresponding peak of the 1H NMR spectrum of the crude product.c Overall yield of the mixture of diastereomers isolated by column chromatography.d 3 Å molecular sieves were used.e 0.5 mmol 1, 0.25 mmol 2a and 30 mol% DBU were used.f H2O (1 equivalent) was used.g nd = not detected. | |||||||
| 1 | DBU | Toluene | 40 | 24 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
45 | — |
| 2 | DBU | Toluene | 60 | 24 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
71 | 8 |
| 3 | DBU | Toluene | 70 | 24 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
59 | 15 |
| 4d | DBU | Toluene | 70 | 24 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
67 | 6 |
| 5e | DBU | Toluene | 70 | 24 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
45 | 27 |
| 6e,f | DBU | Toluene | 70 | 18 | nd | 27 | 35 |
| 7 | DBU | EtOH | 60 | 24 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
15 | — |
| 8g | DBU | MeCN | 60 | 24 | nd | 16 | — |
| 9 | DBU | DMF | 60 | 24 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
30 | 6 |
| 10 | DBU | THF | 60 | 24 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
41 | 5 |
| 11g | Hünig’s base | Toluene | 60 | 24 | nd | >5 | — |
| 12g | DABCO | Toluene | 60 | 24 | nd | 11 | — |
| 13 | K2CO3 | Toluene | 60 | 24 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
22 | 8 |
| 14 | Cs2CO3 | Toluene | 60 | 24 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
27 | 11 |
| 15 | L-Proline | Toluene | 80 | 48 | — | NR | — |
| 16 | Zn(OTf)3 | Toluene | 80 | 36 | — | <5 | — |
| 17 | In(OTf)3 | Toluene | 80 | 36 | — | <10 | — |
| 18g | Yb(OTf)3 | Toluene | 80 | 36 | nd | 21 | — |
| 19g | Gd(OTf)3 | Toluene | 80 | 36 | nd | 19 | — |
Taking the above observations collectively, the rational mechanism for the formation of compounds 3a and 4a has been described in Scheme 2. The Michael adduct 3a is formed via a nucleophilic addition of carbanion 7 (in situ generated from β-keto ester 1) with 2a. For compound 4a, at first, compound 2a may undergo hydrolysis by water in the presence of DBU under heating conditions to form a benzaldehyde which may condense with β-keto ester 1 to form intermediate 8. After that, the Michael addition reaction takes place between carbanion 7 and intermediate 8 to generate 1,5-dicarbonyl compound 9. The latter undergoes intramolecular aldol condensation to provide six-membered cyclic compound 10. Finally, aerobic oxidation of intermediate 11 (the enolic form of 10) may lead to the salicylate derivative 4a.
In view of the above mechanism, we planned the development of an organocatalytic, one-pot, atom-economical and tandem method for the construction of poly-functionalized ortho-phenolic ester scaffolds from the same β-keto ester 1. This core unit is manifested in a large number of biologically potent natural products.12 Thus, several efforts from the synthetic and medicinal chemistry community have been directed towards access to this aromatic backbone.13 Towards this, we have identified two standard procedures (method A and B, Scheme 3) by the combination of β-keto ester 1 with either alkylidene malonates (method A) or aromatic aldehydes (method B) in toluene at 70 °C in the presence of DBU (30 mol%) under aerobic conditions. The obtained results clearly demonstrated that the aryl rings of β-aryl-alkylidene malonates or aromatic aldehydes bearing either electron donating (Me, OMe, OBn) or withdrawing (F, Cl, Br, CN) substituents always led to higher yields of corresponding hexa-substituted salicylates (4a–4n, 59–70% yields, Scheme 3) with β-keto ester 1 by the method B as compared to method A (24–36%).
Next, we turned our attention to establishing the scope and generality of this Michael reaction by using several β-aryl-substituted alkylidene malonates (2a–o) under standard conditions (Table 1, entry 2). The outcome of the results indicated that both electron rich (Me, MeO, BnO) and electron poor (Cl, Br, F, CF3, NO2 and CN) functional groups on the aryl rings of β-aryl-alkylidene malonates (entries 2–13, Table 2) reacted easily with β-keto ester 1 by this procedure. Consequently, all these reactions afforded non-separable diastereomeric mixtures of the corresponding Michael products in good to high combined yields (3a–3m, 67–85%, entries 2–13) with moderate diastereoselectivities (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 80
40 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dr). Interestingly, many chemically sensitive functional groups such as Cl, Br, F, NO2, CN, BnO, MeO, CO2Me, CO2Et etc. were unaffected which provides great opportunities for further alterations of these functional groups. Moreover, alkylidene malonates with hetero-aryl groups, namely thiophene (entry 14) and furan (entry 15), were also found to be good Michael acceptors for this reaction, resulting in the corresponding addition products in high yields of 81% (3n) and 78% respectively with moderate dr values (up to ≤62
20 dr). Interestingly, many chemically sensitive functional groups such as Cl, Br, F, NO2, CN, BnO, MeO, CO2Me, CO2Et etc. were unaffected which provides great opportunities for further alterations of these functional groups. Moreover, alkylidene malonates with hetero-aryl groups, namely thiophene (entry 14) and furan (entry 15), were also found to be good Michael acceptors for this reaction, resulting in the corresponding addition products in high yields of 81% (3n) and 78% respectively with moderate dr values (up to ≤62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38).
38).
| Entry | R1 | T/h | drb | Name | Yieldc (%) |
|---|---|---|---|---|---|
| a All the above reactions were carried out with compound 1 (0.25 mmol), alkylidene malonates (2a–o, 0.3 mmol) and DBU (20 mol%) in dry toluene (0.5 mL) at 60 °C.b Diastereomeric ratio was determined by the corresponding peak of the 1H NMR spectrum of the crude product.c Isolated yield of the mixture of diastereomers after column chromatography. | |||||
| 1 | Ph | 24 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
3a | 71 |
| 2 | 3-MeC6H4 | 23 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
3b | 77 |
| 3 | 4-MeC6H4 | 22 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
3c | 76 |
| 4 | 4-MeOC6H4 | 28 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
3d | 71 |
| 5 | 3-MeO-4-BnOC6H3 | 27 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
3e | 67 |
| 6 | 4-FC6H4 | 26 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3f | 82 |
| 7 | 2-ClC6H4 | 25 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
3g | 80 |
| 8 | 4-ClC6H4 | 25 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
3h | 84 |
| 9 | 3-BrC6H4 | 22 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
3i | 81 |
| 10 | 4-BrC6H4 | 26 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3j | 79 |
| 11 | 4-NCC6H4 | 20 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
3k | 83 |
| 12 | 4-O2NC6H4 | 20 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
3l | 85 |
| 13 | 4-F3CC6H4 | 23 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3m | 74 |
| 14 | 2-Thiophenyl | 22 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
3n | 81 |
| 15 | 2-Furyl | 23 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
3o | 78 |
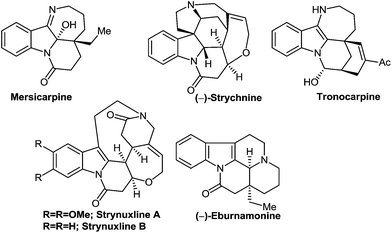
In order to visualize the importance of this methodology, the synthesized Michael adducts may be further altered to highly substituted 8,9-dihydropyrido[1,2-a]indol-6(7H)-one derivatives as this angular tricyclic scaffold is repeatedly found in a large number of naturally occurring indole alkaloids (Fig. 1) and exhibits a broad spectrum of biological activities.14,15 Due to their great applications in synthetic as well as medicinal chemistry, various powerful and innovative approaches have been adopted for the construction of these important N-containing tricyclic compounds in recent years.5c,8a,14 Despite the great success, the diastereoselective synthesis of 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-one derivatives is still lacking. Towards this goal, Michael adduct 3a (62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 dr) was treated with Zn/NH4Cl in THF
38 dr) was treated with Zn/NH4Cl in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C. Pleasingly, after 20 h, we isolated the target 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-one (6a) in 45% yield with excellent diastereoselectivity (92
1) at 80 °C. Pleasingly, after 20 h, we isolated the target 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-one (6a) in 45% yield with excellent diastereoselectivity (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
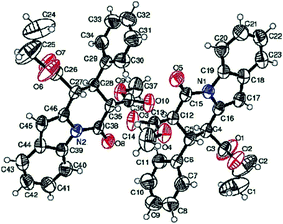
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr) together with 2-indolyl derivative 5a in 30% yield. The relative configuration of major diastereomer 6a was confirmed as trans–trans–trans by single crystal X-ray diffraction data (Fig. 2, details in the ESI†). In order to obtain a better yield and diastereoselectivity, various conditions were tested with Michael adduct 3a as shown in Table 3. It is obvious that the best results were achieved in terms of yield (80%) and diastereoselectivity (95
8 dr) together with 2-indolyl derivative 5a in 30% yield. The relative configuration of major diastereomer 6a was confirmed as trans–trans–trans by single crystal X-ray diffraction data (Fig. 2, details in the ESI†). In order to obtain a better yield and diastereoselectivity, various conditions were tested with Michael adduct 3a as shown in Table 3. It is obvious that the best results were achieved in terms of yield (80%) and diastereoselectivity (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) of compound 6a when the reaction was performed in EtOH
5 dr) of compound 6a when the reaction was performed in EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium at 80 °C using Zn/NH4Cl as a mild reducing agent. The same procedure was applied to other Michael adducts (3c–3n) to prepare the 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-one derivatives possessing three contiguous chiral centers. Typical results are shown in Scheme 4. Importantly, attaching several sensitive functional groups (Me, OMe, OBn, Cl, F, CF3) on the aryl rings of Michael products did not show any problem in this one-pot sequence process (no over-reduction, transesterification etc.) and resulted in clean and complete reduction of the nitro group to an amine, followed by cycloaromatization and subsequent lactamization, providing the corresponding heterocyclic compounds (6c–6m) in good to high yields (62–81%) with excellent diastereoselectivities (up to ≤96
1) medium at 80 °C using Zn/NH4Cl as a mild reducing agent. The same procedure was applied to other Michael adducts (3c–3n) to prepare the 7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-one derivatives possessing three contiguous chiral centers. Typical results are shown in Scheme 4. Importantly, attaching several sensitive functional groups (Me, OMe, OBn, Cl, F, CF3) on the aryl rings of Michael products did not show any problem in this one-pot sequence process (no over-reduction, transesterification etc.) and resulted in clean and complete reduction of the nitro group to an amine, followed by cycloaromatization and subsequent lactamization, providing the corresponding heterocyclic compounds (6c–6m) in good to high yields (62–81%) with excellent diastereoselectivities (up to ≤96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dr). The Michael product with a thiophene substituent afforded the desired pyrido[1,2-a]indolone 6n in 61% yield and 4
4 dr). The Michael product with a thiophene substituent afforded the desired pyrido[1,2-a]indolone 6n in 61% yield and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.
1 dr.
| Entry | Conditions | Yieldc,d (%) | |
|---|---|---|---|
| 5ac | 6ad | ||
| a Reactions were carried out with 3a (0.1 mmol), metal powder (0.3 mmol) and NH4Cl (1.0 mmol) in the specified mixture of solvents (4.5 mL).b Reaction was performed with 3a (0.1 mmol), 10% Pd/C (5.0 mg) and ammonium formate (0.5 mmol) in EtOH (2.0 mL).c Overall yield of mixture of diastereomers 5a.d Isolated yield of major diastereomer of 6a after column chromatography.e Parentheses indicate diastereomeric ratios. | |||
| 1a | Zn, NH4Cl, THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 °C, 20 h 1), 80 °C, 20 h |
30 | 45 (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)e 8)e |
| 2a | Zn, NH4Cl, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 80 °C, 20 h 1) 80 °C, 20 h |
7 | 80 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)e 5)e |
| 3a | Fe, NH4Cl, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 80 °C, 20 h 1) 80 °C, 20 h |
15 | 67 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)e 5)e |
| 4a | In, NH4Cl, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 80 °C, 20 h 1) 80 °C, 20 h |
13 | 71 (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)e 7)e |
| 5b | 10% Pd/C, NH4HCO2, EtOH 80 °C, 20 h | 12 | 65 (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)e 5)e |
| 6b | 10% Pd/C, NH4HCO2, EtOH rt, 3 h | 83 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)e 1)e |
— |
 | ||
| Scheme 4 One-pot diastereoselective synthesis of 7,8,9-trisubstituted 8,9-dihydropyrido[1,2-a]indol-6(7H)-ones. | ||
Conclusions
In summary, we have developed a convenient two-step method for the construction of biologically attractive new functionalized trans–trans–trans-7,8,9-trisubstituted-8,9-dihydropyrido[1,2-a]indol-6(7H)-ones in good to high yields (61–81%) with excellent diastereoselectivities (up to ≤96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 dr). The two-step processes proceed through a Michael reaction between ethyl 4-(2-nitrophenyl)acetoacetate and alkylidene malonate catalyzed by DBU, followed by tandem reductive cycloaromatization–lactamization sequence reaction using Zn/NH4Cl as a mild reducing agent. Interestingly, synthetically challenging hexa-substituted salicylate derivatives could be achieved under aerobic conditions from the same starting materials through a one-pot tandem retro-Knoevenagel–Michael condensation–aromatization process catalyzed by DBU. In addition, method B using benzaldehydes as electrophiles confirms the first step of the mechanism as the retro-Knoevenagel reaction of benzylidene malonate catalysed by DBU. Moreover, the present divergent synthetic approaches are associated with several important features: they are simple, mild, proceed in good to high yields, afford excellent diastereomeric ratios up to ≤96
4 dr). The two-step processes proceed through a Michael reaction between ethyl 4-(2-nitrophenyl)acetoacetate and alkylidene malonate catalyzed by DBU, followed by tandem reductive cycloaromatization–lactamization sequence reaction using Zn/NH4Cl as a mild reducing agent. Interestingly, synthetically challenging hexa-substituted salicylate derivatives could be achieved under aerobic conditions from the same starting materials through a one-pot tandem retro-Knoevenagel–Michael condensation–aromatization process catalyzed by DBU. In addition, method B using benzaldehydes as electrophiles confirms the first step of the mechanism as the retro-Knoevenagel reaction of benzylidene malonate catalysed by DBU. Moreover, the present divergent synthetic approaches are associated with several important features: they are simple, mild, proceed in good to high yields, afford excellent diastereomeric ratios up to ≤96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, have a broad substrate scope, tolerate sensitive functionalities etc. Further endeavours towards the development of the enantioselective synthesis of 7,8,9-trisubstituted-8,9-dihydro[1,2-a]indol-6(7H)-one derivatives as well as applications of these angular tricyclic compounds are under investigation and will be documented in due course.
4, have a broad substrate scope, tolerate sensitive functionalities etc. Further endeavours towards the development of the enantioselective synthesis of 7,8,9-trisubstituted-8,9-dihydro[1,2-a]indol-6(7H)-one derivatives as well as applications of these angular tricyclic compounds are under investigation and will be documented in due course.
Acknowledgements
The authors thank DST (Project no. SB/S1/OC-19/2013) and CSIR (Project no. 02(0019)/11/EMR-II) research grants for the generous financial support. A.S., S.B. and S.S. are also thankful to UGC for their fellowships. Special thanks to the reviewers for their constructive suggestions for improvement of the manuscript.Notes and references
- (a) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon Press, Oxford, 1992 Search PubMed; (b) R. Ballini, G. Bosica, D. Fiorini, A. Palmieri and M. Petrini, Chem. Rev., 2005, 105, 933 CrossRef CAS PubMed; (c) M. P. Sibi and S. Manyem, Tetrahedron, 2000, 56, 8033 CrossRef CAS; (d) M. Sánchez-Roselló, J. L. Aceña, A. Simón-Fuentes and C. del Pozo, Chem. Soc. Rev., 2014, 43, 7430 RSC.
- Review on Michael addition reactions, see: (a) J. Christoffers and A. Baro, Angew. Chem., Int. Ed., 2003, 42, 1688 CrossRef CAS PubMed; (b) O. M. Berner, L. Tedeschi and D. Enders, Eur. J. Org. Chem., 2002, 1877 CrossRef CAS; (c) S. B. Tsogoeva, Eur. J. Org. Chem., 2007, 1701 CrossRef CAS; (d) J. L. Vicario, D. Badia, L. Carillo and E. Reyes, Organocatalytic Enantioselective Conjugate Addition Reactions, The Royal Society of Chemistry, Cambridge, 2010 Search PubMed.
- (a) J. Oelerich and G. Roelfes, Org. Biomol. Chem., 2015, 13, 2793 RSC; (b) S. Duan, R. Jana and J. A. Tunge, J. Org. Chem., 2009, 74, 4612 CrossRef CAS PubMed; (c) Y. Miyake, K. Nakajima and Y. Nishibayashi, J. Am. Chem. Soc., 2012, 134, 3338 CrossRef CAS PubMed; (d) J. Wang, Y. Zhou, L. Zhang, Z. Li, X. Chen and H. Liu, Org. Lett., 2013, 15, 1508 CrossRef CAS PubMed; (e) Y.-H. Shi, Z. Wang, B. Hu, M. Wang, J. S. Fossey and W.-P. Deng, Org. Lett., 2011, 13, 6010 CrossRef CAS PubMed; (f) K. Yamada, M. Maekawa, T. Akindele, M. Nakano, Y. Yamamoto and K. Tomioka, J. Org. Chem., 2008, 73, 9535 CrossRef CAS PubMed; (g) J. Wu, D. Wang, F. Wu and B. Wan, J. Org. Chem., 2013, 78, 5611 CrossRef CAS PubMed; (h) R. Dodda, T. Mandal and C.-G. Zhao, Tetrahedron Lett., 2008, 49, 1899 CrossRef CAS PubMed; (i) D. A. Evans, T. Rovis, M. C. Kozlowski, C. W. Downey and J. S. Tedrow, J. Am. Chem. Soc., 2000, 122, 9134 CrossRef CAS.
- (a) J. M. Betancort, K. Sakthivel, R. Thayumanavan and C. F. Barbas III, Tetrahedron Lett., 2001, 42, 4441 CrossRef CAS; (b) D. R. Magar, C. Chang, Y.-F. Ting and K. Chen, Eur. J. Org. Chem., 2010, 2062 CrossRef CAS; (c) C.-L. Cao, X.-L. Sun, J.-L. Zhou and Y. Tang, J. Org. Chem., 2007, 72, 4073 CrossRef CAS PubMed; (d) J. Liu, Z. Yang, X. Liu, Z. Wang, Y. Liu, S. Bai, L. Lin and X. Feng, Org. Biomol. Chem., 2009, 7, 4120 RSC; (e) R. Chowdhury and S. K. Ghosh, Org. Lett., 2009, 11, 3270 CrossRef CAS PubMed; (f) Y. Yang, S. Dong, X. Liu, L. Lin and X. Feng, Chem. Commun., 2012, 48, 5040 RSC; (g) M. Chiarucci, M. Lombardo, C. Trombini and A. Quintavalla, Adv. Synth. Catal., 2012, 354, 364 CrossRef CAS.
- (a) S. Brandau, A. Landa, J. Franzén, M. Marigo and K. A. Jørgensen, Angew. Chem., Int. Ed., 2006, 45, 4305 CrossRef CAS PubMed; (b) G.-L. Zhao, J. Vesely, J. Sun, K. E. Christensen, C. Bonneau and A. Córdova, Adv. Synth. Catal., 2008, 350, 657 CrossRef CAS; (c) D. Du, L. Li and Z. Wang, J. Org. Chem., 2009, 74, 4379 CrossRef CAS PubMed.
- Y. Liu, S. Shirakawa and K. Maruoka, Org. Lett., 2013, 15, 1230 CrossRef CAS PubMed.
- (a) D. P. Gavin and J. C. Stephens, ARKIVOC, 2013, iv, 76 CrossRef; (b) H. Wang and R. Fan, J. Org. Chem., 2010, 75, 6994 CrossRef CAS PubMed.
- (a) K. Higuchi, S. Suzuki, R. Ueda, N. Oshima, E. Kobayashi, M. Tayu and T. Kawasaki, Org. Lett., 2015, 17, 154 CrossRef CAS PubMed; (b) D. Royer, Y.-S. Wong, S. Plé, A. Chiaroni, K. Diker and J. Lévy, Tetrahedron, 2008, 64, 9607 CrossRef CAS PubMed; (c) R. A. Bunce and B. Nammalwar, J. Heterocycl. Chem., 2009, 46, 172 CrossRef CAS; (d) T. C. Turner, K. Shibayama and D. L. Boger, Org. Lett., 2013, 15, 1100 CrossRef CAS PubMed; (e) C. L. Martin, L. E. Overman and J. M. Rohde, J. Am. Chem. Soc., 2010, 132, 4894 CrossRef CAS PubMed; (f) B. Bradshaw, G. Etxebarria-JardÍ and J. Bonjoch, Org. Biomol. Chem., 2008, 6, 772 RSC.
- B. Bradshaw, C. Parra and J. Bonjoch, Org. Lett., 2013, 15, 2461 Search PubMed.
- (a) S. Singh, A. Srivastava and S. Samanta, Tetrahedron Lett., 2012, 53, 6087 CrossRef CAS PubMed; (b) S. Biswas, P. K. Jaiswal, S. Singh, M. S. Mobin and S. Samanta, Org. Biomol. Chem., 2013, 11, 7084 RSC; (c) P. K. Jaiswal, S. Biswas, S. Singh and S. Samanta, Org. Biomol. Chem., 2013, 11, 8410 RSC; (d) S. Biswas, D. Majee, A. Dagar and S. Samanta, Synlett, 2014, 2115 CAS; (e) S. Singh, A. Srivastava, S. M. Mobin and S. Samanta, RSC Adv., 2015, 5, 5010 RSC; (f) D. Majee, S. Biswas, S. M. Mobin and S. Samanta, Tetrahedron Lett., 2014, 55, 4553 CrossRef CAS PubMed; (g) P. K. Jaiswal, S. Biswas, B. Pathak, S. M. Mobin and S. Samanta, RSC Adv., 2013, 3, 10644 RSC.
- A solution of alkylidene malonate (2a, 2j and 2k), H2O (1.0 equiv.) and DBU (30 mol%) in toluene was heated at 70 °C for 18 h. The corresponding aldehydes were formed in 35–45%.
- (a) J. H. P. Tyman, Synthetic and Natural Phenols, Elsevier, New York, 1996 Search PubMed; (b) L. Yet, Chem. Rev., 2003, 103, 4283 CrossRef CAS PubMed; (c) C.-L. Chen, F.-L. Liu, C.-C. Lee, T.-C. Chen, A. A. A. Ali, H.-K. Sytwu, D.-M. Chang and H.-S. Huang, J. Med. Chem., 2014, 57, 8072 CrossRef CAS PubMed; (d) C. Ito, M. Itoigawa, Y. Mishina, H. Tomiyasu, M. Litaudon, J.-P. Cosson, T. Mukainaka, H. Tokuda, H. Nishino and H. Furukawa, J. Nat. Prod., 2001, 64, 147 CrossRef CAS.
- Synthesis of salicylate derivatives, see: (a) W. T. Teo, W. Rao, C. J. H. Ng, S. W. Y. Koh and P. W. H. Chan, Org. Lett., 2014, 16, 1248 CrossRef CAS PubMed; (b) J. Cordes, S. Laclef, A. J. P. White and A. G. M. Barlett, J. Org. Chem., 2012, 77, 3524 CrossRef CAS PubMed; (c) A. Sharma, J. Pandey and R. P. Tripathi, Tetrahedron Lett., 2009, 50, 1812 CrossRef CAS PubMed; (d) Q. Gao, X.-C. Tang, Y.-M. Pan, H.-S. Wang and Y. Liang, Chem. Commun., 2012, 48, 12080 RSC; (e) M. Fouche, L. Rooney and A. G. M. Barrett, J. Org. Chem., 2012, 77, 3060 CrossRef CAS PubMed.
- Hydropyrido[1,2-a]indole synthesis, see: (a) D. V. Patil, M. A. Cavitt, G. Paul and S. France, Chem. Commun., 2011, 47, 10278 RSC; (b) A. Biechy and S. Z. Zard, Org. Lett., 2009, 11, 2800 CrossRef CAS PubMed; (c) A. Umehara, H. Ueda and H. Tokuyama, Org. Lett., 2014, 16, 2526 CrossRef CAS PubMed; (d) R. Nakajima, T. Ogino, S. Yokoshima and T. Fukuyama, J. Am. Chem. Soc., 2010, 132, 1236 CrossRef CAS PubMed; (e) J. Magolan and M. A. Kerr, Org. Lett., 2006, 8, 4561 CrossRef CAS PubMed; (f) Y. Fu, Y. Zhang, H. He, L. Hou, Y. Di, S. Li, X. Luo and X. Hao, J. Nat. Prod., 2012, 75, 1987 CrossRef CAS PubMed; (g) G. Li, X. Huang and L. Zhang, Angew. Chem., Int. Ed., 2008, 47, 346 CrossRef CAS PubMed; (h) S. Zhou and Y. Jia, Org. Lett., 2014, 16, 3416 CrossRef CAS PubMed.
- (a) M. Kato, S. Nishino, K. Ito and H. Takasugi, Chem. Pharm. Bull., 1995, 43, 1346 CrossRef CAS; (b) D. L. Taylor, P. S. Ahmed, P. Chambers, A. S. Tyms, J. Bedard, J. Duchaine, G. Falardeau, J. F. Lavallée, W. Brown, R. F. Rando and T. Bowlin, Antiviral Chem. Chemother., 1999, 10, 79 CAS; (c) M. V. Riofski, J. P. John, M. M. Zheng, J. Kirshner and D. A. Colby, J. Org. Chem., 2011, 76, 3676 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data of new compounds. CCDC 1045133. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra01430a |
| This journal is © The Royal Society of Chemistry 2015 |