Superhydrophobic Ti6Al4V surfaces with regular array patterns for anti-icing applications
Abstract
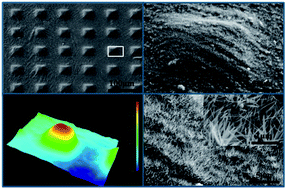
In this article, we present a route to fabricate a robust anti-icing superhydrophobic surface containing the hierarchical structures of microscale array patterns (built by micromachining) and nanohairs (prepared via hydrothermal growth) on a Ti6Al4V substrate. In particular, the superhydrophobic surfaces not only exhibited high non-wettability and water repellency, but also generated a tremendous anti-icing potential. The results of the measurements indicated that the apparent contact angle reached 160°, the contact angle hysteresis reduced to 2°, and the spreading and recoiling process of an impact droplet can be completed within 12 ms. Furthermore, it also caused a longer icing-delay time (approximately 765 s) to hinder the ice formation and growth at −10 °C, and the ice adhesion strength was also only 70 kPa.

 Please wait while we load your content...
Please wait while we load your content...