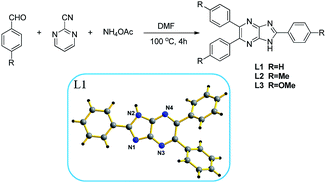
Synthesis and characterization on novel fluorescent sensors for Pd2+/Pd0 with high selectivity†
You Zi,
Hua Meng,
Xue-Qiang Chu,
Xiao-Ping Xu* and
Shun-Jun Ji*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, 215123, People's Republic of China. E-mail: xuxp@suda.edu.cn; shunjun@suda.edu.cn; Fax: +86-512-65880307; Tel: +86-512-65880307
First published on 6th March 2015
Abstract
Novel fluorescent sensors L1–L3 based on triaryl 1H-imidazo[4,5-b]pyrazine have been synthesized through a new and simple route from inexpensive and readily available materials in one pot. They exhibited high selectivity for palladium detection (oxidation states of 0 and +2) based on the fluorescence quenching effect.
Introduction
With the development of industrial, agricultural, and urban modernization in the last several decades, the pollution of heavy metals (HMs), used widely in agriculture, chemical and industrial processes, is causing serious threats to living organisms.1 As one of heavy metals (HMs), palladium plays a key role in various fields, such as electrical and electronic industries, dental crowns, automobiles, jewellery and catalysts.2 In particular, palladium-catalyzed organic reactions, namely Suzuki–Miyaura, Heck, Sonogashira and Buchwald–Hartwig, are increasingly important because of their advantages in the synthesis of complex molecules.3 However, with the wide use of the palladium, a high level of residual palladium is often found in the final product.4 It is well known that palladium can bind to thiol-containing amino acids, proteins, DNA and other biomolecules and thereby may seriously influence our health in an adverse way.5 As a consequence, much attention has been paid to the development of efficient methods for detecting palladium species (mainly Pd0 and Pd2+).Conventional methods for the selective detection of palladium include atomic absorption spectroscopy (AAS), inductively coupled plasma atomic emission spectroscopy (ICP-AES), solid-phase micro extraction-high performance liquid chromatography (SPME-HPLC), X-ray fluorescence (XRF), etc.6 In spite of their rapid and extremely sensitive analysis, they require complicated sample preparation procedures, rigorous experimental conditions, expensive and sophisticated instrumentation and experienced operators.7 Thus, considerable attention has been paid to fluorescent methods for the detection of palladium because of their apparent advantages, such as low cost, operational simplicity, high sensitivity and selectivity, over other methods.8 Meanwhile, the most desirable fluorescent sensors should possess sensitive manner by dramatic change in emission color and/or intensity.
The mechanisms for detection methods are mostly based on the chemical reactions between Pd and the detector (cycle opening of rhodamine derivatives or cleavage reactions etc.) or complexation of Pd with the detector.8–12 As a strong fluorescence quencher, Pd2+ ions or Pd0 could be detected through fluorescence quenching.10,11 But less molecules were proved to be highly selective sensors (see ESI† for details).10 Usually, fluorescent sensing systems for Pd2+ species work through the coordination of sulfur or alkynyl groups to palladium.10,12 However, other competitive ions such as Hg2+ and Pt2+ would decrease the efficiency of detection.8p,10b Therefore, it's meaningful to discover new fluorescent sensing systems with novel structure and high anti-interference. Although imidazole skeleton has been used for the detection of series of ions,13 no report, to the best of our knowledge, has been covered for the detection of Pd2+/Pd0 with imidazo[4,5-b]pyrazine derivatives.
Herein, we devoted our attention to the construction of triaryl 1H-imidazo[4,5-b]pyrazine derivatives and presented novel compounds L1–L3 (L1 was determined by X-ray diffraction) as new fluorescent sensors that are capable of reporting Pd2+/Pd0 with distinct fluorescence quenching. The detailed synthetic method for the preparation of L1–L3 is shown in Scheme 1. The reaction of benzaldehydes, 2-cyanopyrimidine and ammonium acetate in the solution of DMF at 110 °C for 4 h give the sensors. (And a plausible mechanism has been provided in ESI.†)
Results and discussion
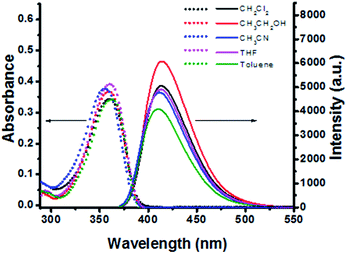
The spectroscopic properties of sensor L1 were measured in different dilute solutions (10−5 M). As shown in Fig. 1, L1 had an absorption wavelength with a maximum at about 360 nm and had slight changes in different solutions. Moreover, the fluorescence spectrum shows that L1 can emit intense fluorescence at 410 nm in series of solutions and the fluorescence quantum yield (QY) of L1 in CH3CN was calculated to be 0.23, with tryptophan as the standard.14To validate the selectivity of L1 in practice, the absorption and fluorescence spectra of L1 in the presence of various metal ions have been studied. Different metal species had little effect on the absorption of sensor L1 (Fig. S1, ESI†). Fluorescence spectral data revealed that L1 selectively responded to Pd2+ resulting in a serious fluorescence quenching (Fig. 2A), which could be easily observed by the naked eye (Fig. 2B), and the fluorescence quantum yield (QY) decreased to 0.05. Among other metal sources examined, little emission intensifying was observed upon addition of Cu2+, Cd2+, Ni2+, Co2+, Ba2+, Zn2+, Sn2+, Ca2+, Mg2+, K+, Na+, Li+, Pb2+, Fe3+, Ru3+, Hg2+ and Pt2+ (Fig. 2A). Thus, according to the obvious spectroscopy changes, the designed L1 as the fluorescence sensor can detect Pd2+ with high selectivity.
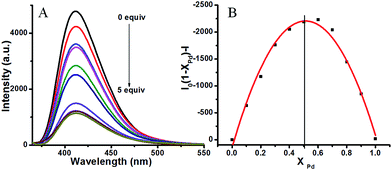
Fig. 3A displays the changes to the emission spectra of L1 in the presence of different concentrations of Pd2+ and it can be seen that the fluorescence intensity of the emission maximum at 410 nm decreased linearly with the increase of Pd2+ concentration. The Job's plot15 (Fig. 3B) revealed that L1 formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometrical complex with Pd2+.
1 stoichiometrical complex with Pd2+.
As the absorption spectra of L1 displayed no obvious red-shift after the addition of Pd2+ ions which might indicate that the mode of linkage between the ligand and the Pd2+ is mostly intrachain instead of interchain.9c,10d The binding mode of L1–Pd2+ is illustrated in Scheme 2. The solution of L1 displayed an intense fluorescence before the addition of Pd2+ ion, and owing to the charge transfer (CT) from the sensor to the metal after the complexation with Pd2+, a serious fluorescence quenching occurred.
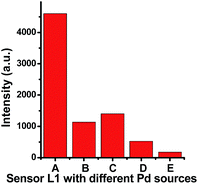
To study the selectivity of sensor L1 toward other palladium species, a series of palladium complexes with different initial oxidation states or anions such as Pd(dba)2, PdCl2(dppf) and Pd(OAc)2, were introduced and examined (Fig. 4). Interestingly, fluorescence of L1 could be similarly quenched by these palladium species, indicating that the response process depends on palladium metal itself, while not the oxidation state, ligands and anions.
As is well known, an important property of a sensor is high selectivity toward the analyte in the presence of other competitive species. To further investigate the selectivity of sensor L1 for Pd2+ ion, competition experiments were performed in the presence of Pd2+ (3 equiv.) mixed with each of the guest cations (10 equiv.) namely Cu2+, Cd2+, Ni2+, Co2+, Ba2+, Zn2+, Sn2+, Ca2+, Mg2+, K+, Na+, Li+, Pb2+, Fe3+, Ru3+, Hg2+ and Pt2+, respectively. According to Fig. 2A and 5, different degree of emission intensification was observed upon addition of miscellaneous competitive metal ions while the fluorescence exhibited serious quenching in the presence of Pd2+ for each system. This result indicated that sensor L1 displayed a high selectivity and anti-interference toward palladium species when coexisting with other competitive metal ions.
In addition, the effect of pH on the fluorescence of sensor L1 in the absence and presence of Pd2+ ion was then evaluated in the pH range from 3.1 to 10.2. As shown in Fig. 6, there was little effect on the fluorescence intensity of sensor L1 under acidic or neutral conditions (pH ≤ 7.0). However, the fluorescence intensity of L1 was rapidly decreased when the conditions turned basic. To our delight, the fluorescence of L1 exhibited serious quenching in the presence of Pd2+ ion under different pH value conditions. The results indicate that sensor L1 could successfully allow Pd2+ detection in a wide pH range.
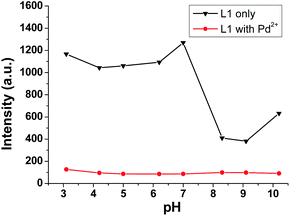 | ||
| Fig. 6 The fluorescence spectra of L1 (10−5 M) and L1 in the presence of 5 equiv. of Pd2+ ion in H2O under different pH conditions. Excitation wavelength was 360 nm. | ||
As the analogs of sensor L1, L2 and L3 were also synthesized (Scheme 1) to examine the influence of molecule structure on the detection of Pd2+. We repeated the detecting experiments under the same conditions (Fig. 7). The wavelength of both absorption and fluorescence of L3 (376 nm/445 nm) had an obvious red-shift due to the better electron donating property of methoxyl (Fig. S2 and S3, ESI†). It's quite clear that both L2 and L3 exhibited fluorescence quenching in the presence of Pd2+ (Fig. 7A and B). Although the degree of quenching was not obvious for L3 compared with the other two sensors, a distinction could still be recognized relative to other competitive ions. At the same time, different initial oxidation states of palladium species were examined (Fig. 7C and D). According to the fluorescence intensity, L2 and L3 could respond to different Pd species as well. The results above revealed that both L2 and L3 are also nice fluorescence sensors for Pd species detection with well selectivity.
Conclusions
In summary, a series of novel fluorescent sensors based on triaryl 1H-imidazo[4,5-b]pyrazine for Pd2+/Pd0 was designed and synthesized. They can be obtained through a new and simple method from inexpensive materials in one pot. The new fluorescent sensor L1 displayed a high selectivity and anti-interference toward palladium species detection in CH3CN solution. The optical spectra had been investigated and the fluorescence of L1 exhibited serious quenching in the presence of palladium species, QY changing from 0.23 to 0.05, while different degree of emission intensifying was observed upon addition of each competitive metal ions. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between the sensor and Pd2+ had been elucidated through the Job's plot curve. Sensors L2 and L3 were obtained and exhibited similar properties to L1 on the detection of Pd species. For the high selectivity and anti-interference toward palladium species detection, sensors L1–L3 were expected for the potential applications in human health and environmental protection.
1 binding stoichiometry between the sensor and Pd2+ had been elucidated through the Job's plot curve. Sensors L2 and L3 were obtained and exhibited similar properties to L1 on the detection of Pd species. For the high selectivity and anti-interference toward palladium species detection, sensors L1–L3 were expected for the potential applications in human health and environmental protection.
Acknowledgements
We gratefully acknowledge the Natural Science Foundation of China (no. 21372174), the Young National Natural Science Foundation of China (no. 21202113), the Ph.D. Programs Foundation of Ministry of Education of China (2013201130004), PAPD.Notes and references
- (a) M. R. Senger, E. P. Rico, M. D. Arizi, A. P. G. Frazzon, R. D. Dias, M. R. Bogo and C. D. Bonan, Toxicology, 2006, 226 Search PubMed; (b) E. M. Nolan and S. J. Lippard, Acc. Chem. Res., 2009, 42, 193 CrossRef CAS PubMed; (c) Y. Ding, Y. Xie, X. Li, J. P. Hill, W. Zhang and W. Zhu, Chem. Commun., 2011, 47, 5431 RSC.
- (a) J. L. Bars, U. Specht, J. S. Bradley and D. G. Blackmond, Langmuir, 1999, 15, 7621 CrossRef; (b) T. Iwasawa, M. Tokunaga, Y. Obora and Y. Tsuji, J. Am. Chem. Soc., 2004, 126, 6554 CrossRef CAS PubMed; (c) M. Lafrance and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 16496 CrossRef CAS PubMed.
- (a) S. L. Buchwald, C. Mauger, G. Mignani and U. Scholzc, Adv. Synth. Catal., 2006, 348, 23 CrossRef CAS; (b) X. Chen, K. M. Engle, D. H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (c) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS PubMed; (d) L. F. Tietze, H. Ila and H. P. Bell, Chem. Rev., 2004, 104, 3453 CrossRef CAS PubMed; (e) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (f) J. Tsuji and T. Mandai, Angew. Chem., Int. Ed., 1995, 34, 2589 CrossRef CAS.
- D. G. Cho and J. L. Sessler, Chem. Soc. Rev., 2009, 38, 1647 RSC.
- (a) T. Z. Liu, S. D. Lee and R. S. Bhatnagar, Toxicol. Lett., 1979, 4, 469 CrossRef CAS; (b) J. C. Wataha and C. T. Hanks, J. Oral Rehabil., 1996, 23, 309 CrossRef CAS PubMed; (c) International Programme on Chemical Safety, Palladium, Environmental Health Criteria Series 226, World Health Organization, Geneva, 2002 Search PubMed; (d) J. Kielhorn, C. Melber, D. Keller and I. Mangelsdorf, Int. J. Hyg. Environ. Health, 2002, 205, 417 CrossRef CAS PubMed.
- (a) C. Locatelli, D. Melucci and G. Torsi, Anal. Bioanal. Chem., 2005, 382, 1567 CrossRef CAS PubMed; (b) K. Van Meel, A. Smekens, M. Behets, P. Kazandjian and R. VanGrieken, Anal. Chem., 2007, 79, 6383 CrossRef CAS PubMed; (c) B. Liu, Y. Y. Bao, H. Wang, F. F. Du, J. Tian, Q. B. Li, T. S. Wang and R. K. Bai, J. Mater. Chem., 2012, 22, 3555 RSC; (d) B. Dimitrova, K. Benkhedda, E. Ivanova and F. Adams, J. Anal. At. Spectrom., 2004, 19, 1394 RSC.
- N. Jakubowski, I. Feldmann and D. Stuewer, J. Anal. At. Spectrom., 1997, 12, 151 RSC.
- (a) D. Kalny, A. M. Albrecht-Gary and J. Havel, Anal. Chim. Acta, 2001, 439, 101 CrossRef CAS; (b) J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780 CrossRef CAS PubMed; (c) S. E. Angell, C. W. Rogers, Y. Zhang, M. O. Wolf and W. E. Jones Jr, Coord. Chem. Rev., 2006, 250, 1829 CrossRef CAS PubMed; (d) Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192 CrossRef CAS PubMed; (e) S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef PubMed; (f) I. Shin, K. H. Ahn, M. Santra and S. K. Ko, Chem. Commun., 2010, 3964 Search PubMed; (g) R. M. F. Bastista, E. Oliveira, S. P. G. Costa, C. Lodeiro and M. M. M. Raposo, Tetrahedron, 2011, 67, 7106 CrossRef PubMed; (h) H. N. Kim, Z. Q. Guo, W. H. Zhu, J. Y. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79 RSC; (i) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS PubMed; (j) L. Zhang, Y. Wang, J. Yu, G. Zhang, X. Cai, Y. Wu and L. Wang, Tetrahedron Lett., 2013, 54, 4019 CrossRef CAS PubMed; (k) G. Zhang, Y. Wen, C. Guo, J. Xu, B. Lu, X. Duan, H. He and J. Yang, Anal. Chim. Acta, 2013, 805, 87 CrossRef CAS PubMed; (l) X. Chen, H. Li, L. Jin and B. Yin, Tetrahedron Lett., 2014, 55, 2537 CrossRef CAS PubMed; (m) A. L. Garner, F. Song and K. Koide, J. Am. Chem. Soc., 2009, 131, 5163 CrossRef CAS PubMed; (n) A. L. Garner and K. Koide, Chem. Commun., 2009, 86 RSC; (o) X. J. Peng, H. L. Li, J. L. Fan, F. L. Song, H. Zhu, J. J. Du and S. G. Sun, Chem.–Eur. J., 2010, 16, 12349 CrossRef PubMed; (p) S. Goswami, D. Sen, N. K. Das, H. K. Fun and C. K. Quah, Chem. Commun., 2011, 47, 9101 RSC; (q) K. H. Ahn and M. E. Jun, Org. Lett., 2010, 12, 2790 CrossRef PubMed; (r) F. Song, A. L. Garner and K. Koide, J. Am. Chem. Soc., 2007, 129, 12354 CrossRef CAS PubMed; (s) H. Li, J. Fan, J. Du, K. Guo, S. Sun, X. Liu and X. Peng, Chem. Commun., 2010, 46, 1079 RSC; (t) H. Li, J. Fan and X. Peng, Chem. Soc. Rev., 2013, 42, 7943 RSC; (u) J. Du, M. Hu, J. Fan and X. Peng, Chem. Soc. Rev., 2012, 41, 4511 RSC; (v) J. Du, J. Fan, X. Peng, P. Sun, J. Wang, H. Li and S. Sun, Org. Lett., 2010, 12, 476 CrossRef CAS PubMed; (w) H. Li, J. Fan, J. Wang, M. Tian, J. Du, S. Sun, P. Sun and X. Peng, Chem. Commun., 2009, 5904 RSC; (x) J. Fan, J. Lu, H. Li, X. Liu, H. Zhu and X. Peng, Chin. J. Appl. Chem., 2011, 28, 1292 CAS; (y) Q. Huang, Y. Zhou, Q. Zhang, E. Wang, Y. Min, H. Qiao, J. Zhang and T. Ma, Sens. Actuators, B, 2015, 208, 22 CrossRef CAS PubMed.
- (a) G. Sivaraman, T. Anand and D. Chellappa, ChemPlusChem, 2014, 79, 1761 CAS; (b) T. Anand, G. Sivaraman, A. Mahesh and D. Chellappa, Anal. Chim. Acta, 2015, 853, 596 CrossRef CAS PubMed; (c) G. Sivaraman, T. Anand and D. Chellappa, RSC Adv., 2012, 2, 10605 RSC; (d) G. Sivaraman, B. Vidya and D. Chellappa, RSC Adv., 2014, 4, 30828 RSC; (e) T. Anand, G. Sivaraman and D. Chellappa, J. Photochem. Photobiol., A, 2014, 281, 47 CrossRef CAS PubMed.
- (a) T. Schwarze, H. Muller, C. Dosche, T. Klamroth, W. Mickler, A. Kelling, H. G. Lohmannsroben, P. Saalfrank and H. J. Holdt, Angew. Chem., Int. Ed., 2007, 46, 1671 CrossRef CAS PubMed; (b) L. P. Duan, Y. F. Xu and X. H. Qian, Chem. Commun., 2008, 6339 RSC; (c) H. Huang, K. Wang, W. Tan, D. An, X. Yang, S. Huang, Q. Zhai, L. Zhou and Y. Jin, Angew. Chem., Int. Ed., 2004, 43, 5635 CrossRef CAS PubMed; (d) B. Liu, Y. Bao, F. Du, H. Wang, J. Tian and R. Bai, Chem. Commun., 2011, 47, 1731 RSC.
- (a) E. Unterreitmaier and M. Schuster, Anal. Chim. Acta, 1995, 309, 339 CrossRef CAS; (b) K. Kubo, Y. Miyazaki, K. Akutso and T. Sakurai, Heterocycles, 1999, 51, 965 CrossRef CAS PubMed; (c) B. K. Pal and M. S. Rahman, Mikrochim. Acta, 1999, 131, 139 CrossRef CAS; (d) Y. J. Fang, H. Chen, Z. X. Gao and X. Y. Jin, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2002, 41, 521 Search PubMed.
- (a) R. J. T. Houk, K. J. Wallace, H. S. Hewage and E. V. Anslyn, Tetrahedron, 2008, 64, 8271 CrossRef CAS PubMed; (b) T. Schwarze, C. Dosche, R. Flehr, T. Klamroth, H. G. Lohmannsroben, P. Saalfrank, E. Cleve, H. J. Buschmanne and H. J. Holdt, Chem. Commun., 2010, 46, 2034 RSC.
- (a) J. Wang, W. Lin and W. Li, Chem.–Eur. J., 2012, 18, 13629 CrossRef CAS PubMed; (b) P. Zhang, B. Shi, T. B. Wei, Y. M. Zhang, Q. Lin, H. Yao and X. M. You, Dyes Pigm., 2013, 99, 857 CrossRef CAS PubMed; (c) S. Huang, Z. Li, S. Li, J. Yin and S. Liu, Dyes Pigm., 2012, 92, 961 CrossRef CAS PubMed; (d) M. Wang, J. Wang, W. Xue and A. Wu, Dyes Pigm., 2013, 97, 475 CrossRef CAS PubMed; (e) M. Ishida, P. Kim, J. Choi, J. Yoon, D. Kim and J. L. Sessler, Chem. Commun., 2013, 49, 6950 RSC.
- E. P. Kirby and R. F. Steiner, J. Phys. Chem., 1970, 74, 4480 CrossRef CAS.
- F. Chen, F. Hou, L. Huang, J. Cheng, H. Liu, P. Xi, D. Bai and Z. Zeng, Dyes Pigm., 2013, 98, 146 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1028080. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra01343g |
| This journal is © The Royal Society of Chemistry 2015 |