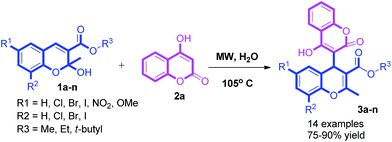
“On water” synthesis of highly functionalized 4H-chromenes via carbon–carbon bond formation under microwave irradiation and their antibacterial properties†
L. Chandrasekhara Raoa,
N. Satish Kumara,
V. Dileepkumarb,
U. S. N. Murthyb and
H. M. Meshram*a
aMedicinal Chemisty and Pharmacology Division, CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad – 500 007, India. E-mail: hmmeshram@yahoo.com; Fax: +91-40-27160512; Tel: +91-40-27191640
bBiology Division CSIR-Indian Institute of Chemical Technology, Tarnaka, Hyderabad – 500 007, India
First published on 13th March 2015
Abstract
An ecofriendly and catalyst free synthesis of novel hybrid molecules of chromene and coumarin is described by C–C bond formation in an aqueous medium under microwave irradiation. The methodology provides cleaner conversion, a shorter reaction time and high regio selectivity which makes the protocol globally recognized. For the first time the reaction between a special type of allylic alcohols 2-hydroxy-2H-chromens and 4-hydroxy coumarin to produce novel functionalised 4H-chromenes in excellent yields was achieved and we evaluated their antibacterial properties.
Introduction
4H-Chromene derivatives were found to exhibit a wide spectrum of biological activities like antimicrobial,1 antiproliferation,2 antitumor,3 antiallergenic,4 antioxidant5 and considerable central nervous system activities.6 Especially HA-14 and EPC2407 were found to exhibit strong cytotoxicity against human cancer cells. On the other hand 4-hydroxy coumarin is a useful building block in many natural products with significant biological activities including analgesic,7 anti-arthritis,8 anti-inflammatory,9 anti-pyretic,10 anti-bacterial,11 anti-viral,12 and anti-cancer13 properties. Due to the distinct biological properties of individual skeletons, we envisioned that the combination of both the structures in a single molecule may enhance the biological activity (Fig. 1).Recently microwave assisted reactions14 have attracted chemists due to their unique advantageous like rate acceleration, short reaction time and improved product yield. Therefore, this technique considered as a promising green approach in drug discovery and development processes. In recent years, many researchers have been focused on developing clean and green protocols15 for the synthesis of various organic compounds. Water has unique properties like ready availability, non toxic nature, and safety in handling. However, after the description of rate acceleration in Claisen rearrangements16 and Diels–Alder reactions17 using water as reaction medium and consequently introduction of “on water concept” by Sharpless et al.18 Owing to the poor solubility of organic compounds in water, it has been used occasionally as a solvent in organic reactions. However, physical and chemical properties of water are altered at elevated temperatures under microwave irradiation in such a way that it behaves both as a pseudo organic solvent and a phase transfer catalyst too.19
The carbon–carbon bond formation reaction is one of the most valuable protocols for the construction of the hybrid molecular framework in organic synthesis.20 However, the catalytic activation of alcohols is difficult due to the low lability of the hydroxy group. The C–C coupling reactions between allylic21 or benzylic22 alcohols and active methylene of arenes have demonstrated successful. 2-Hydroxy-2H-chromene is a special type of allylic alcohol and the reactivity this moiety is associated with its identity as a hemiacetal, thereby allowing the formation of a stable oxonium electrophile, prompted us to develop new methods. Recently, three component reaction has been reported23 for the synthesis of 4-hydroxy coumarin substituted 4H-chromene using ZnO nano particle. To the best our knowledge the reaction of 2-hydroxy 2H-chromene with 4-hydroxy coumarin is not known. In continuation of our work, for the synthesis of novel molecules by environmentally benign reactions24 and to expand the chromene library, herein we report the first synthesis of novel hybrid molecules of chromene and coumarin via C–C bond formation in aqueous medium under microwave irradiation and their antibacterial activity was evaluated (Scheme 1).
Results and discussion
Initially we started the reaction of 2H-chromene (1a), with 4-hydroxy coumarin (2a) in water under microwave irradiation. The reaction proceeded well and after work up 3a was the sole product which was confirmed by spectroscopic data (Mass, NMR, IR and HRMS). Next we focused our study to optimize reaction conditions. Among the different screened solvents, water was found as a suitable solvent for maximum conversion and the results summarized in Table 1. The other solvents like PEG-400, N,N-dimethylformamide, dimethylsulfoxide and ethanol gave low yield. Whereas the reaction did not proceed in solvents like, 1,2-dichloroethane, acetonitrile and tetrahydrofuran. The reaction in neat condition under microwave irradiation led to an inseparable mixture of products. Later to improve the yield of product, we examined different acid catalysts like BF3·OEt2, InBr3, Sc(OTf)3, TiCl4, Anhy. ZnCl2, SnCl4, p-toluenesulfonic acid, iodine under microwave irradiation in suitable solvents which gave lower yield. Among with the various catalysts and solvent systems tested, we observed that water mediated reactions were the most effective in terms of yields.| Entry | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 2H-chromene (1a) (1 mmol), and 4-hydroxy coumarin (1 mmol) in 2 mL of solvent for 20 min under microwave irradiation.b Isolated yield of pure product.c No product formation. | |||
| 1 | EtOH | 90 | 15 |
| 2 | THF | 90 | NRc |
| 3 | CH3CN | 90 | NRc |
| 4 | DCE | 110 | NRc |
| 5 | DMF | 110 | 25 |
| 6 | DMSO | 110 | 33 |
| 7 | PEG-400 | 110 | 54 |
| 8 | H2O | 80 | 43 |
| 9 | H2O | 105 | 78 |
| 10 | H2O | 120 | 54 |
| 11 | — | 110 | 15 |
We next studied the electronic effect of different substituents at 6 and 8 positions on 2H-chromenes. Chromene bearing electron donating groups (OMe) or chromene without any substituent produce low yields. Whereas chromene bearing electron withdrawing substituents (NO2, 6,8 di-halogen) resulted excellent yield of product. Later we also studied the reactivities of chromenes bearing ester group at 3rd position. Chromene bearing t-butyl ester at 3rd position gave the desired product in good yields whereas methyl and ethyl ester containing chromenes produced relatively low yields (Table 2).
All the newly synthesized scaffolds were screened for their in vitro antimicrobial activity against the bacterial strains Staphylococcus aureus (MTCC 96), Staphylococcus epidermidis (MTCC 9041), Bacillus subtilis (MTCC 441), (Gram-positive organisms), Escherichia coli (MTCC 443), Pseudomonas aeruginosa (MTCC 741), Klebsiella pneumonia (MTCC 618), (Gram-negative organisms) by agar well diffusion method.25 Solutions were prepared by dissolving the compound in DMSO and different concentrations were made. After inoculation, wells were scooped out with 3 mm sterile cork borer and the lids of the dishes were replaced. To each well different concentrations of test solutions were added. Controls were maintained. The treated and the controls were kept at 37 °C for 24 h incubator. Inhibition zones were measured, the diameter calculated in millimeter and the corresponding results were tabulated. The antibiotic penicillin and streptomycin are used as reference antibacterial substances, respectively for comparison. The bacterial zone of inhibition values is shown in Table 3.
| Entry | S. epidermidis | S. aureus | B. subtilis | E. coli | P. aeruginosa | K. pneumoniae |
|---|---|---|---|---|---|---|
| a —No activity. aConcentration 1.0 mg mL−1. bValues, including diameter of the well (8 mm), are means of three replicates. | ||||||
| 3a | — | — | — | — | — | — |
| 3b | — | — | — | — | — | — |
| 3c | 13 | 11 | — | — | — | — |
| 3d | 14 | 13 | — | — | 12 | — |
| 3e | 11 | 11 | — | — | 9 | — |
| 3f | 12 | 12 | — | — | — | — |
| 3g | 14 | 11 | — | — | 9 | — |
| 3h | — | 14 | — | — | — | — |
| 3i | — | 10 | — | — | — | — |
| 3j | 13 | — | — | — | — | — |
| 3k | 14 | — | 15 | — | — | — |
| 3l | 19 | 21 | — | — | 19 | — |
| 3m | 16 | — | 14 | — | — | — |
| 3n | — | — | — | — | — | — |
The minimum inhibitory concentrations (MIC) of the synthetic derivatives, by microdilution method recommended by CLSI Standard Protocol26 in liquid medium (nutrient agar) distributed in 96-well plates, serial dilutions of the tested compounds were performed (concentrations from 150 μg mL−1 to 0.97 μg mL−1) in a 200 μL culture medium final volume, afterwards each well was seeded with a 50 μL microbial suspension of 0.5 MacFarland density. For each test a microbial culture control and a sterility control (negative) were performed. The plates were incubated at 37 °C for 24 hours. The lowest concentration which inhibited the visible microbial growth was considered the MIC (μg mL−1) value for the tested compound. Penicillin and streptomycin were used as reference drugs. The minimum inhibitory concentration (MIC) values are presented in the Table 4.
| Compound | Minimum inhibitory concentration | |||||
|---|---|---|---|---|---|---|
| Gram positive organisms | Gram negative organisms | |||||
| Staphylococcus aureus (MTCC96) | Staphylococcus epidermidis (MTCC9041) | Bacillus subtilis (MTCC441) | Escherichia coli (MTCC443) | Pseudomonas aeruginosa (MTCC741) | Klebsiella pneumonia (MTCC618) | |
| 3a | >150 | >150 | >150 | >150 | >150 | >150 |
| 3b | >150 | >150 | >150 | >150 | >150 | >150 |
| 3c | 37.5 | 18.75 | >150 | >150 | >150 | >150 |
| 3d | 18.75 | 18.75 | >150 | >150 | 18.75 | >150 |
| 3e | 37.5 | 37.5 | >150 | >150 | 37.5 | >150 |
| 3f | 18.75 | 18.75 | >150 | >150 | >150 | >150 |
| 3g | 37.5 | 18.75 | >150 | >150 | 37.5 | >150 |
| 3h | 18.75 | >150 | >150 | >150 | >150 | >150 |
| 3i | 18.75 | >150 | >150 | >150 | >150 | >150 |
| 3j | >150 | 18.75 | >150 | >150 | >150 | >150 |
| 3k | >150 | 18.75 | 18.75 | >150 | >150 | >150 |
| 3l | 9.3 | 9.3 | >150 | >150 | 9.3 | >150 |
| 3m | >150 | 18.75 | 18.75 | >150 | >150 | >150 |
| 3n | >150 | >150 | >150 | >150 | >150 | >150 |
| Penicillin | 1.562 | 3.125 | 1.562 | 12.5 | 12.5 | 6.25 |
| Streptomycin | 6.25 | 3.125 | 6.25 | 6.25 | 1.562 | 3.125 |
The compounds were screened for their in vitro antibacterial activity. The experimental results of antimicrobial activity indicated a variable degree of efficacy of the compounds against different strains of bacteria. Compound 3l showed very strong activity (9.375 lg mL−1), against S. aureus, S. epidermidis, and P. aeruginosa. Whereas compounds 3d, 3e and 3g showed significant activity against S. aureus, S. epidermidis, and P. aeruginosa (18.75 μg mL−1). The compounds 3c and 3f showed significant activity against S. aureus and S. epidermidis. The compounds 3k and 3m showed significant activity against S. epidermidis and Bacillus subtilis (18.75 μg mL−1). Whereas compounds 3h and 3i showed significant activity against S. aureus only. Compound 3k showed significant activity against S. epidermidis (18.75 μg mL−1) only. They did not show any considerable effect on other strains (>150 μg mL−1) as indicated in Table 4. The substitution at 6 and 8 positions on 4H-chromene moiety with Cl, Br, dichloro and diiodo have shown more activity, when compared to NO2, OMe substitutions at 6 position. Chromenes without any substitution did not show any activity. 4H-Chromenes with t-butyl ester have shown more activity when compared with chromenes containing methyl and ethyl esters.
Conclusion
In conclusion, we have developed a highly efficient and catalyst-free synthesis of 4-hydroxy coumarin substituted 4H-chromenes in aqueous medium under microwave irradiation. We first time synthesized novel coumarin substituted 4H-chromenes. Simple reaction conditions, easy isolation of the products, use of green solvent are the advantages of the present method. Moreover the present protocol provides rapid and easy access for the synthesis of a large number of 4H-chromenes in quantitative yields which may find application in organic synthesis. In addition to we reported antibacterial properties of all the synthesized products which may find applications in medicinal chemistry.Experimental section
General information
All the chemicals were purchased from Sigma Aldrich and Alpha Aesar company and used without further purification as received. All 1H and 13C NMR spectra were recorded in CDCl3 on Avance 300 or Avance 500 spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual CHCl3 (1H: δ 7.26 ppm, 13C: δ 77.00 ppm) as an internal reference. Coupling constants (J) are reported in Hertz (Hz). Peak multiplicity is indicated as follows: s—singlet, d—doublet, t—triplet, q—quartet, m—multiplet and dd—doublet of doublet. Melting points were measured on a BUCHI melting point machine. IR spectra were recorded on Thermo Nicolet FT/IR-5700 spectrometer. Mass spectra were recorded using Waters mass spectrometer. High resolution mass spectrums (HRMS) were recorded using Applied Bio-Sciences HRMS spectrometer at national center for mass spectroscopy-IICT.General procedure for the synthesis of 4H-chromene derivatives (3a–3n)
In a microwave reaction vial 2H-chromene (1a) (1 mmol) and 4-hydroxy coumarin (2a) (1 mmol) in 2 mL of water kept under microwave irradiation for 20 minutes at 105 °C as mentioned in Table 1. After completion of the reaction (indicated by TLC), the free flowing solid was filtered and washed with water (20 mL) to afford the desired products as pale yellow solids. The product thus obtained was recrystallized from ethanol to get pure compounds as white or pale yellow crystals. The isolated compounds were well characterized by IR, 1H NMR, 13C NMR and HRMS.Ethyl-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3a)
White solid; Mp 148–150 °C; IR: νmax 3238, 2877, 1682, 1612, 1502, 1485, 1376, 1231, 1151, 1046, 880, 751 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.00 (d, J = 7.55 Hz, 1H), 7.63 (s, 1H), 7.42–7.53 (m, 1H), 7.06–7.31 (m, 4H), 6.88–7.00 (2H, m), 5.54 (s, 1H), 4.00–4.19 (m, 2H), 2.45 (s, 3H), 1.20 (t, J = 7.18 Hz, 3H), 13C NMR (75 MHz, CDCl3): δ 167.2, 161.3, 151.8, 149.7, 130.7, 127.4, 126.8, 123.2, 122.9, 122.6, 121.7, 115.9, 115.4, 114.6, 108.9, 100.7, 59.5, 30.4, 19.2, 13.4; m/z (ESI); 379 [M + H]+, 401 [M + Na]+.Tert-butyl-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3b)
White solid; Mp 148–150 °C; IR: νmax 3219, 2917, 1667, 1608, 1512, 1477, 1363, 1262, 1135, 1051, 895, 761 cm−1; 1H NMR (300 MHz, CDCl3): δ 10.74 (s, 1H), 7.98 (d, J = 8.31 Hz, 1H), 7.44–7.52 (m, 1H), 7.13–7.32 (m, 3H), 6.94–7.06 (3H, m), 5.12 (s, 1H), 2.46 (s, 3H), 1.60 (s, 9H), 13C NMR (75 MHz, CDCl3): δ 170.6, 164.0, 161.2, 160.2, 153.0, 151.3, 131.5, 127.9, 124.2, 124.1, 123.5, 121.8, 116.1, 115.4, 114.6, 109.8, 102.0, 83.3, 30.1, 28.3, 21.3; m/z (ESI); 407 [M + H]+, 429 [M + Na]+. HRMS calcd for C24H22O6Na: 429.13150, found: 429.13086.Ethyl-6′-chloro-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3c)
White solid; Mp 148–150 °C; IR: νmax 3162, 2875, 1709, 1675, 1607, 1465, 1231, 1202, 1085, 974, 751 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.00 (d, J = 7.93 Hz, 1H), 7.44–7.53 (m, 1H), 7.38 (s, 1H), 7.19–7.39 (m, 2H), 7.03–7.13 (m, 2H), 6.92 (d, J = 8.50 Hz, 1H), 5.39 (s, 1H), 4.07–4.26 (m, 2H), 2.46 (s, 3H), 1.25 (t, J = 7.93 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 166.6, 161.2, 160.9, 159.6, 151.8, 148.4, 130.8, 127.4, 127.1, 126.7, 123.0, 122.8, 116.1, 115.8, 115.4, 108.3, 100.6, 59.4, 30.2, 18.3, 13.3; m/z (ESI); 413 [M + H]+, 435 [M + Na]+. HRMS calcd for C22H17O6ClNa: 435.06039, found: 435.06059.Tert-butyl-6′-chloro-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3d)
White solid; Mp 148–150 °C; IR: νmax 3182, 2916, 1707, 1673, 1618, 1445, 1376, 1258, 1236, 1036, 927, 749 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.02 (d, J = 7.36 Hz, 1H), 7.56 (s, 1H), 7.47–7.55 (m, 1H), 7.21–7.32 (m, 2H), 7.05–7.11 (m, 2H), 6.90 (d, J = 9.25 Hz, 1H), 5.42 (s, 1H), 2.40 (s, 3H), 1.41 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 164.6, 157.9, 150.8, 147.4, 130.1, 126.2, 126.1, 125.8, 123.4, 122.1, 115.4, 114.8, 114.6, 78.6, 29.4, 26.5, 17.7; m/z (ESI); 441 [M + H]+, 463 [M + Na]+.Methyl-6′-bromo-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3e)
White solid; Mp 148–150 °C; IR: νmax 3218, 2929, 1717, 1675, 1621, 1572, 1485, 1376, 1212, 1046, 915, 751 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.02 (d, J = 8.12 Hz, 1H), 7.62 (s, 1H), 7.46–7.55 (m, 1H), 7.22–7.32 (m, 4H), 6.86 (d, J = 8.69 Hz, 1H), 5.53 (s, 1H), 3.66 (s, 3H), 2.44 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 167.6, 161.5, 159.1, 149.2, 131.1, 130.2, 129.9, 124.4, 123.0, 116.7, 116.0, 115.7, 115.4, 108.5, 100.6, 51.0, 30.5, 19.4 m/z (ESI); 443 [M + H]+, 465 [M + Na]+.Ethyl-6-bromo-4-(4-hydroxy-2-oxo-2H-chromen-3-yl)-2-methylchroman-3-carboxylate (3f)
White solid; Mp 148–150 °C; IR: νmax 3214, 2925, 1715, 1673, 1624, 1567, 1481, 1397, 1206, 1064, 988, 754 cm−1; 1H NMR (300 MHz, CDCl3): δ 10.36 (br-s, 1H), 7.93 (d, J = 7.93 Hz, 1H), 7.47–7.52 (m, 1H), 7.25–7.31 (m, 3H), 7.22 (d, J = 8.24 Hz, 1H), 7.11 (d, J = 2.14 Hz, 1H), 6.91 (d, J = 8.70 Hz, 1H), 5.10 (s, 1H), 4.23–4.34 (m, 2H), 2.48 (s, 3H), 1.34 (t, J = 7.17 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 171.0, 164.5, 161.1, 160.4, 152.9, 150.4, 131.8, 130.9, 130.5, 124.2, 123.8, 123.7, 117.2, 116.6, 116.5, 116.2, 109.2, 100.6, 62.2, 31.9, 21.0, 14.2 m/z (ESI); 457 [M + H]+, 479 [M + Na]+. HRMS calcd for C24H16O6BrNa: 479.01056, found: 479.01248.Tert-butyl-6′-bromo-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3g)
White solid; Mp 148–150 °C; IR: νmax 3209, 2917, 1707, 1636, 1566, 1465, 1376, 1213, 1058, 955, 756 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.07 (s, 1H), 8.03 (d, J = 7.74 Hz, 1H), 7.51–7.59 (m, 1H), 7.22–7.34 (m, 3H), 7.20 (d, J = 2.27 Hz, 1H), 6.88 (d, J = 8.50 Hz, 1H), 5.48 (s, 1H), 2.34 (s, 3H), 1.33 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 169.9, 163.9, 157.1, 150.4, 147.4, 135.6, 129.9, 128.6, 128.4, 123.6, 121.8, 115.6, 114.4, 113.3, 101.2, 78.0, 28.8, 26.0, 17.2; m/z (ESI); 485 [M + H]+, 507 [M + Na]+. HRMS calcd for C24H21O6BrNa: 507.04295, found: 507.04135.Methyl-4-hydroxy-2′-methyl-6′-nitro-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3h)
Pale yellow solid; Mp 148–150 °C; IR: νmax 3208, 2926, 1703, 1672, 1623, 1524, 1339, 1242, 1206, 1065, 988, 759 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.99–8.09 (m, 3H), 7.65 (br-s, 1H), 7.48–7.56 (m, 1H), 7.26–7.33 (m, 1H), 7.22 (d, J = 8.12 Hz, 1H), 7.10 (d, J = 9.44 Hz, 1H), 5.66 (s, 1H), 3.68 (s, 3H), 2.47 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.8, 159.5, 153.9, 151.4, 143.3, 130.6, 123.1, 122.9, 122.5, 122.3, 115.2, 115.1, 115.0, 107.2, 100.8, 50.2, 29.6, 18.2; m/z (ESI); 432 [M + Na]+.Ethyl-4-hydroxy-2′-methyl-6′-nitro-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3i)
Pale yellow solid; Mp 148–150 °C; IR: νmax 3212, 2927, 1702, 1675, 1624, 1522, 1346, 1245, 1208, 1056, 975, 756 cm−1; 1H NMR (300 MHz, CDCl3): δ 10.23–10.52 (br-s, 1H), 8.08 (dd, J1 = 9.06 Hz, J2 = 2.64 Hz, 1H), 8.01 (dd, J1 = 7.93 Hz, J2 = 1.32 Hz, 1H), 7.93 (d, J = 2.45 Hz, 1H), 7.48–7.56 (m, 1H), 7.28–7.35 (m, 1H), 7.21 (d, J = 8.31 Hz, 1H), 7.14 (d, J = 8.88 Hz, 1H), 5.17 (s, 1H), 4.25–4.39 (m, 2H), 2.53 (s, 2H), 1.36 (t, J = 7.17 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 170.6, 163.7, 161.3, 161.0, 155.7, 152.9, 143.9, 132.2, 124.4, 124.0, 122.9, 116.4, 116.2, 143.9, 132.2, 124.0, 122.9, 116.4, 116.2, 108.8, 101.5, 62.5, 31.9, 20.8, 14.1; m/z (ESI); 424 [M + H]+, 446 [M + Na]+. HRMS calcd for C22H18O8N: 424.10319, found: 424.10269.Methyl-6′,8′-dichloro-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3j)
White solid; Mp 148–150 °C; IR: νmax 3153, 1718, 1683, 1607, 1559, 1461, 1237, 1201, 1058, 975, 756 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.03 (d, J = 7.93 Hz, 1H), 7.82 (br-s, 1H), 7.50–7.56 (m, 1H), 7.20–7.31 (m, 3H), 7.01 (d, J = 2.54 Hz, 1H), 5.52 (s, 1H), 4.05–4.21 (m, 2H), 2.50 (s, 3H), 1.22 (t, J = 6.99 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 165.7, 159.5, 151.3, 144.0, 130.6, 126.6, 126.5, 125.2, 124.9, 122.6, 122.4, 119.8, 115.2, 115.0, 107.3, 100.7, 50.1, 29.9, 18.1; m/z (ESI); 433 [M + H]+, 455 [M + Na]+. HRMS calcd for C21H14O6Cl2Na: 455.00601, found: 429.00596.Ethyl-6′,8′-dichloro-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3k)
White solid; Mp 148–150 °C; IR: νmax 3150, 1717, 1681, 1568, 1475, 1243, 1207, 1076, 965, 753 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.01 (d, J = 7.93 Hz, 1H), 7.47–7.55 (m, 1H), 7.46 (br-s, 1H), 7.22–7.31 (m, 2H), 7.19 (d, J = 2.45 Hz, 1H), 7.03 (d, J = 2.45 Hz, 1H), 5.57 (s, 1H), 3.64 (s, 3H), 2.47 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 166.6, 160.6, 152.1, 144.8, 131.2, 127.5, 127.3, 125.9, 125.5, 123.1, 120.6, 115.8, 108.4, 101.6, 59.9, 30.8, 18.9, 13.5; m/z (ESI); 447 [M + H]+, 469 [M + Na]+. HRMS calcd for C22H16O6Cl2Na: 469.02214, found: 469.02161.Tert-butyl-6′,8′-dichloro-4-hydroxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3l)
White solid; Mp 148–150 °C; IR: νmax 3148, 1715, 1676, 1542, 1455, 1239, 1198, 1081, 974, 757 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.04 (d, J = 6.98 Hz, 1H), 8.01 (s, 1H), 7.51–7.58 (m, 1H), 7.23–7.33 (m, 3H), 7.03 (d, J = 2.26 Hz, 1H), 5.51 (s, 1H), 2.39 (s, 3H), 1.35 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 164.1, 157.6, 151.0, 143.8, 130.4, 126.2, 124.9, 122.3, 119.6, 114.9, 114.8, 102.3, 79.1, 30.0, 26.6, 17.7; m/z (ESI); 475 [M + H]+, 497 [M + Na]+.Tert-butyl-4-hydroxy-6′,8′-diiodo-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3m)
White solid; Mp 148–150 °C; IR: νmax 3292, 2966, 2922, 1691, 1668, 1623, 1446, 1396, 1254, 1201, 1065, 900, 755 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.04 (d, J = 7.74 Hz, 1H), 7.89–7.96 (br-s, 1H), 7.84 (d, J = 1.70 Hz, 1H), 7.50–7.58 (m, 1H), 7.37 (s, 1H), 7.23–7.33 (m, 2H), 5.50 (s, 1H), 2.40 (s, 3H), 1.36 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 165.3, 160.0, 151.6, 148.9, 143.4, 136.0, 130.8, 125.4, 122.8, 122.7, 115.5, 115.4, 108.2, 85.9, 84.1, 79.9, 30.6, 27.2, 18.5; m/z (ESI); 659 [M + H]+, 681 [M + Na]+. HRMS calcd for C24H21O6I2: 658.94300, found: 680.92480.Tert-butyl-4-hydroxy-6′-methoxy-2′-methyl-2-oxo-2H,4′H-[3,4′-bichromene]-3′-carboxylate (3n)
White solid; Mp 148–150 °C; IR: νmax 3203, 2973, 1675, 1619, 1561, 1498, 1226, 1163, 1065, 992, 763 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.98 (dd, J1 = 7.93 Hz, J2 = 1.53 Hz, 1H), 7.46–7.50 (m, 1H), 7.27–7.30 (m, 1H), 7.21 (d, J = 8.39 Hz, 1H), 6.96 (d, J = 9.00 Hz, 1H), 6.92 (d, J = 8.50 Hz, 1H), 6.72 (dd, J1 = 9.00 Hz, J2 = 2.90 Hz, 1H), 6.48 (d, J = 2.90 Hz, 1H), 5.10 (s, 1H), 3.70 (s, 3H), 2.44 (s, 3H), 1.52 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 170.7, 164.3, 161.2, 160.3, 156.1, 152.9, 145.6, 131.5, 124.1, 123.5, 122.6, 116.9, 116.2, 113.6, 112.3, 109.7, 101.2, 83.2, 55.6, 32.5, 28.3, 21.4; m/z (ESI); 459 [M + Na]+. HRMS calcd for C25H24O7Na: 459.14241, found: 459.14142.Acknowledgements
The authors thank CSIR, New Delhi, for financial support as part of XII. Five Year Plan Program under the title ACT (CSC-0301) and to Dr A. Kamal, an outstanding scientist and Head of MCP Division, for his support and encouragement. L.C.R. and N.S.K. thank CSIR for the award of a fellowship.Notes and references
- (a) A. M. El-Agrody, M. H. El-Hakium, M. S. A. El-Latif, A. H. Fekry, E. S. M. El-Sayed and K. A. El-Gareab, Acta Pharm., 2000, 50, 111 CAS; (b) M. C. Yimdjo, A. G. Azebaze, A. E. Nkengfack, M. Meyer, B. Bodo and Z. T. Fomum, Phytochemistry, 2004, 65, 2789 CrossRef CAS PubMed; (c) Z. Q. Xu, K. Pupek, W. J. Suling, L. Enache and M. T. Flavin, Bioorg. Med. Chem., 2006, 14, 4610 CrossRef CAS PubMed; (d) V. Jeso and K. C. Nicolaou, Tetrahedron Lett., 2009, 50, 1161 CrossRef CAS PubMed.
- M. Brunavs, C. P. Dell, P. T. Gallagher, W. M. Owton and C. W. Smith, Eur. Pat. Appl., EP 557075 A1 19930825, 1993.
- S. J. Mohr, M. A. Chirigos, F. S. Fuhrman and J. W. Pryor, Cancer Res., 1975, 35, 3750 CAS.
- K. Gorlitzer, A. Dehne and E. Engler, Arch. Pharm., 1983, 316, 264 CrossRef CAS.
- (a) L. Alvey, S. Prado, V. Huteau, B. Saint-Joanis, S. Michel, M. Koch, S. T. Cole, F. Tillequin and Y. L. Janin, Bioorg. Med. Chem., 2008, 16, 8264 CrossRef CAS PubMed; (b) T. Symeonidis, M. Chamilos, D. J. Hadjipavlou-Litina, M. Kallitsakis and K. E. Litinas, Bioorg. Med. Chem. Lett., 2009, 19, 1139 CrossRef CAS PubMed.
- F. Eiden and F. Denk, Arch. Pharm., 1991, 324, 353 CrossRef CAS.
- E. Adami, E. Marazzi-Uberti and C. Turba, Arch. Ital. Sci. Farmacol., 1959, 9, 61 CAS.
- D. Chiarino, G. C. Grancini, V. Frigeni and A. Carenzi, Eur. Pat. Appl., EP 284017, 1988.
- A. C. Luchini, P. Rodrigues-Orsi, S. H. Cestari, L. N. Seito, A. Witaicenis, C. H. Pellizzon and L. C. D. Stasi, Biol. Pharm. Bull., 2008, 31, 1343 CAS.
- P. Stern, M. Dezelic and R. Kosak, Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol., 1957, 232, 356 (Molecules, 2009, 14, 4801) CrossRef CAS.
- Z. H. Chohan, A. U. Shaikh, A. Rauf and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2006, 21, 741 CrossRef CAS PubMed.
- B. S. Kirkiacharian, E. Clercq, R. Kurkjian and C. Pannecouque, J. Pharm. Chem., 2008, 42, 265 CrossRef CAS PubMed.
- M. A. Velasco-Velázquez, J. Agramonte-Hevia, D. Barrera, A. Jiménez-Orozco, M. J. García-Mondragón, N. Mendoza-Patiño, A. Landa and J. Mandoki, Cancer Lett., 2003, 198, 179 CrossRef.
- (a) M. Y. Stevens, K. Wieckowski, P. Wu, R. T. Savant and L. R. Odell, Org. Biomol. Chem., 2015, 13, 2044 RSC; (b) S. Karamtulla, S. Pal, M. N. Khan and L. H. Choudhury, RSC Adv., 2014, 4, 37889 RSC; (c) L. F. X. Feng, J. J. Zhang, J. D. Hu, Z. Xun, J. J. Wang, Z. B. Huang and D. Q. Shi, Green Chem., 2015, 17, 1535 RSC; (d) M. Marnozzi, S. Tondi, G. Marcelli and G. Giorgi, Tetrahedron, 2014, 70, 9485 CrossRef PubMed; (e) P. Appukkuttan and E. V. D. Eycken, Eur. J. Org. Chem., 2008, 1133 CrossRef CAS; (f) M. Hosseini, N. Stiasni and V. Barbieri, J. Org. Chem., 2007, 72, 1417 CrossRef CAS PubMed.
- (a) W. Ye, Y. Li, L. Zhou, J. Liu and C. Wang, Green Chem., 2015, 17, 188 RSC; (b) Y. Xie, C. Xiufang, S. Liu, H. Chen, W. Zhou, L. Yang and G. J. Deng, Green Chem., 2015, 17, 209 RSC.
- (a) W. N. White and E. F. Wolfarth, J. Org. Chem., 1970, 35, 2196 CrossRef CAS; (b) R. M. Coates, B. D. Rogers, S. J. Hobbs, D. R. Peck and D. P. Curran, J. Am. Chem. Soc., 1987, 109, 1160 CrossRef CAS; (c) J. J. Gajewski, J. Jurayj, D. R. Kimbrough, M. E. Gande, B. Ganem and B. K. Carpenter, J. Am. Chem. Soc., 1987, 109, 1170 CrossRef CAS; (d) E. Brandes, P. A. Grieco and J. J. Gajewski, J. Org. Chem., 1989, 54, 515 CrossRef CAS.
- (a) O. Diels and K. Alder, Ann. Chem., 1931, 490, 243 CrossRef; (b) R. B. Woodward and H. Baer, J. Am. Chem. Soc., 1948, 70, 1161 CrossRef CAS; (c) L. C. Lane and C. H. J. Parker, US Pat., 2444, 115, 1948; (d) T. A. Eggelte, H. De Koning and H. O. Huisman, Tetrahedron, 1973, 29, 2491 CrossRef CAS; (e) D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolbe and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed.
- (a) P. Lidstrom, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225 CrossRef CAS; (b) Y. Ju and R. S. Varma, Green Chem., 2004, 6, 219 RSC.
- (a) S. Shirakawa and S. Kobayashi, Org. Lett., 2007, 9, 311 CrossRef CAS PubMed; (b) C. R. Piao, Y. L. Zhao, X. D. Han and Q. Liu, J. Org. Chem., 2008, 73, 2264 CrossRef CAS PubMed.
- G. Kaur, M. Kausik and S. Trehan, Tetrahedron Lett., 1997, 38, 2521 CrossRef CAS.
- (a) S. K. Xiang, L. H. Zhang and N. Jiao, Chem. Commun., 2009, 6487 RSC; (b) M. Mirza-Aghayan, R. Boukherroub and M. Rahimifard, Tetrahedron Lett., 2009, 50, 5930 CrossRef CAS PubMed; (c) A. Bunge, H. J. Hamann, E. McCalmont and J. Liebscher, Tetrahedron Lett., 2009, 50, 4629 CrossRef CAS PubMed; (d) F. Shi, M. K. Tse, X. J. Cui, D. Gördes, D. Michalik, K. Thurow, Y. Q. Deng and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 5912 CrossRef CAS PubMed; (e) R. Ahmad, A. Riahi and P. Langer, Tetrahedron Lett., 2009, 50, 1490 CrossRef CAS PubMed.
- P. P. Ghosh and A. R. Das, J. Org. Chem., 2013, 78, 6170 CrossRef CAS PubMed.
- (a) H. M. Meshram, P. N. Reddy, P. V. Murthy and J. S. Yadav, Synth. Commun., 2007, 37, 4117 CrossRef CAS; (b) H. M. Meshram, N. Nageswara Rao, L. Chandrasekhara Rao and N. Satish Kumar, Tetrahedron Lett., 2012, 53, 3963 CrossRef CAS PubMed; (c) H. M. Meshram, N. Nageswara Rao, L. Chandrasekhara Rao and N. Satish Kumar, Der Pharma Chemica, 2012, 4, 1355–1360 Search PubMed; (d) L. Chandrasekhara Rao, H. M. Meshram, N. Satish Kumar, N. Nageswara Rao and N. Jagadeesh Babu, Tetrahedron Lett., 2014, 55, 1127 CrossRef CAS PubMed; (e) L. Chandrasekhara Rao, N. Satish Kumar, N. Jagadeesh babu and H. M. Meshram, Tetrahedron Lett., 2014, 55, 5342 CrossRef CAS PubMed; (f) P. B. Thakur and H. M. Meshram, RSC Adv., 2014, 4, 6019 RSC; (g) P. B. Thakur and H. M. Meshram, RSC Adv., 2014, 4, 5343 RSC.
- M. E. Linday, Practical Introduction to Microbiology, E and F.N. Spon Ltd., United Kingdom, 1962, p. 177 Search PubMed.
- P Clinical And Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility Tests, Eighteen informational supplement M100-S18, 2008.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01301a |
| This journal is © The Royal Society of Chemistry 2015 |