Self-assembly of L-cysteine–gold nanoparticles as chiral probes for visual recognition of 3,4-dihydroxyphenylalanine enantiomers†
Lin Zhang,
Chunli Xu,
Guoxin Song and
Baoxin Li*
Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an 710062, China. E-mail: libaoxin@snnu.edu.cn; Fax: +86-29-81530727
First published on 11th March 2015
Abstract
A simple protocol to distinguish enantiomers is extremely intriguing and useful. Herein, we report on a method for the visual recognition of 3,4-dihydroxyphenylalanine (Dopa) enantiomers. It is based on the chirality of L-cysteine-capped gold nanoparticles (L-Cys-capped AuNPs) that can be used as a chiral selector for L- and D- forms of Dopa. On addition of L-Dopa to a solution of the L-Cys-capped AuNPs, a color change from red to blue can be seen, while no color change is found on addition of D-Dopa. The chiral recognition can be achieved by eye and simple spectrophotometry. Notably, this method does not require complicated chiral modification. The method excels through its low-cost, good availability of materials, and its simplicity.
Introduction
As the most available therapeutic of Parkinson's disease, 3,4-dihydroxy-L-phenylalanine (L-Dopa) is a biological precursor of a neurotransmitter in the brain and can convert into dopamine once it is taken up in a living organism.1 The absence of L-Dopa will cause the depletion of dopamine in the brain, resulting in muscle stiffness, postural instability and so on. Therefore, L-Dopa plays a very crucial role in clinics and neurochemistry. However, as its antipode, D-Dopa is not only inactive, but also toxic.2 Owing to different metabolisms of the active and inactive components, using a racemic mixture containing L- and D-Dopa may cause serious side effects.3 Therefore, chiral recognition of Dopa enantiomers is essential in chemical, biological and pharmaceutical industries. Currently, chiral separation techniques, high-performance liquid chromatography4,5 and capillary electrophoresis,6–8 are most frequently used to distinguishing Dopa enantiomers. However, most of them are usually time-consuming and require expensive chiral columns. In recent years, chiral selectors were used to modify the electrodes, and some electrochemical methods were developed for recognition of Dopa enantiomers without the need of separation.9–13 Although progress in chiral discrimination of Dopa has been achieved during the past decades,4–13 it is still a challenge to develop a much simple, inexpensive and convenient techniques for chiral recognition and quantification of Dopa. One of the most pressing challenges in the design of chiral assay is to achieve visual discrimination of enantiomers.14 An ideal assay that achieves this would have to translate an enantioselective molecular recognition event into an appreciable color change.15Colorimetric assay has attracted much attention because of its low cost, simplicity, and practicality. Since color changes can be read out by the bare eye, colorimetric analysis does not require expensive or sophisticated instrumentation and can be applied to field analysis.16 With recent developments in nanoscience and nanotechnology, new methods of designing colorimetric assays are emerging. The well-dispersed gold nanoparticles (AuNPs) solution is red, whereas the aggregated AuNPs solution appears a blue color. The color change induced by aggregation of AuNPs provides an ideal platform for colorimetric analysis. Over the past few decades, a large number of AuNPs-based colorimetric assays have been used to detect small molecules, DNA and proteins.17,18 However the reports of chiral sensing system using AuNPs as colorimetric probes are few in number. Lately, Li group bond N-acetyl-L-cysteine, a kind of chiral molecule, onto the surface of AuNPs to acquire N-acetyl-L-cysteine-capped AuNPs, which are able to colorimetric recognition of chiral tyrosine,19 and Han group used L-proline-capped AuNPs to colorimetric chiral discrimination of histidine.20 To achieve the goal of chiral recognition, the functionalization of AuNPs with appropriate chiral ligand is critical and essential.
Herein, L-cysteine (L-Cys), one chiral thiol molecule, was self-assembled on the surface of AuNPs to obtain L-Cys-capped AuNPs (L-Cys–AuNPs). We designed a simple and visual method for chiral recognition of Dopa using L-Cys–AuNPs as colorimetric probes (Scheme 1). In the presence of L-Dopa, a noticeable red-to-blue color change of L-Cys–AuNPs solution can be observed, whereas no color change is found in the presence of D-Dopa. Compared to the recently published method on voltammetric discrimination of D- and L-Dopa using a glassy carbon electrode modified with β-cyclodextrin, multiwalled carbon nanotubes and ionic liquid,10 this method is more attractive because of low cost, ready availability and simple manipulation because the assay described in this work is easily readout with the naked eye or using a UV-vis spectrometer.
 | ||
| Scheme 1 Schematic illustration of visual chiral recognition of L- and D-Dopa using L-Cys–AuNPs as colorimetric probes. | ||
Experimental
Reagents and materials
Chloroauric acid (HAuCl4) was purchased from Shanghai Chemical Reagent Company (Shanghai, China). L-Cysteine was purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China). Enantiomerically pure Dopa purchased from Sigma-Aldrich (St. Louis, MO). Other reagents were of analytical grade and directly used without further purification. Ultrapure water was prepared by a Milli-Q system (Millipore, France) and used in all experiments.Instrumentation
UV-vis absorption spectra were recorded on a UV-3900H UV-vis Spectrophotometer (Shimadzu, Japan) at room temperature. The photographs were taken with a Cannon 500 digital camera. The pH measurements were carried out on model PB-10 digital ion analyzer (Sartorius Scientific instruments Co., Ltd., China, Beijing). Transmission electron microscopy (TEM) measurements were made on a JEM-2100 transmission electron microscope (JeolCo. Ltd., Japan). The samples for TEM characterization were prepared by placing a drop of colloidal solution on carbon-coated copper grid and dried at room temperature. The circular dichroism (CD) spectra were performed on a Chirascan CD spectrophotometer (Applied Photophysics, Leatherhead, UK), of which the lamp was always kept under a stable stream of dry purified nitrogen (99.99%) during experiments. The FTIR spectra were acquired on a Tensor 27™ FTIR spectrometer system (Bruker Optics, Milan, Italy). The zeta potential measurements were performed on a Zetasizer Nano-ZS90 (Malvern Instrument, Worcs, UK).Synthesis and characterization of L-cysteine-capped AuNPs
All glassware and magnetic stirrer bars used in the following procedure was thoroughly cleaned in aquaregia (HNO3/HCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), rinsed thoroughly in water, and then oven-dried prior to use, to avoid unwanted nucleation during the synthesis, as well as aggregation of gold colloid solution. AuNPs were prepared according to the published protocol.21 Firstly, 5.6 mL sodium citrate solution (1%) was rapidly added into a boiled HAuCl4 solution (50 mL, 0.04%) under vigorous stirring. The mixed solution was boiled for 10 min, and further stirred for 15 min. The resulting solution was cooled to room temperature and filtered.
3, v/v), rinsed thoroughly in water, and then oven-dried prior to use, to avoid unwanted nucleation during the synthesis, as well as aggregation of gold colloid solution. AuNPs were prepared according to the published protocol.21 Firstly, 5.6 mL sodium citrate solution (1%) was rapidly added into a boiled HAuCl4 solution (50 mL, 0.04%) under vigorous stirring. The mixed solution was boiled for 10 min, and further stirred for 15 min. The resulting solution was cooled to room temperature and filtered.
A quantity of 5 mL above AuNPs solution was transferred into a 20 mL glass vial, and 100 μL of 2 × 10−4 M L-cysteine solution was added dropwise to the AuNPs solution under stirring. After the mixture was stirred for 2 h at room temperature (ca. 20 °C), the L-cysteine-capped AuNPs were prepared. The newly synthesized AuNPs was stored at 4 °C in an opaque glass container and ready for use. The synthesized L-Cys–AuNPs were characterized by using UV-vis spectroscopy and TEM in order to measure the size and concentration of AuNPs. The synthesized L-Cys–AuNPs solution was 17.6 nM, which was estimated using Beer's law and an extinction coefficient of 2.7 × 108 M−1 cm−1 at 520 nm for 13 nm AuNPs.22
Colorimetric chiral discrimination
A typical colorimetric chiral assay was realized by following the procedure given in Scheme 1. To a 1.5 mL Eppendorf tube were in succession added 100 μL of L-Cys–AuNPs (17.6 nM), 300 μL of pH 4.0 Britton–Robinson (BR) buffer (0.04 M H3PO4, 0.04 M HAc, 0.04 M H3BO3), and 50 μL of L-Dopa or D-Dopa with the appropriate concentration (in the range of 1–2000 μM), and then the mixed solution was incubated for 15 min at room temperature (ca. 20 °C). Finally, the pictures were taken, and the UV-vis spectra were recorded.Results and discussion
Characterization of L-cysteine-capped AuNPs
In this system, L-cysteine is chosen as chiral candidate for modifying the AuNPs due to its chiral structure. On the other hand, L-cysteine can easily assemble on the surface of AuNPs via a solid Au–S covalent bond. The synthesized L-Cys–AuNPs were characterized by UV-vis spectrum, FT-IR spectrum and TEM. There was only one absorption band at 520 nm in UV-vis spectrum (Fig. S1†), which originates from the surface plasmon absorption of the dispersed AuNPs.23 In order to verify L-cysteine binding to the AuNPs surface, we measured the FT-IR spectra of L-cysteine and L-cysteine-modified AuNPs (Fig. 1). It was note the IR spectrum of L-cysteine exhibit the spectra characteristic of a typical amino acid. In addition, a weak band near 2550 cm−1 virtually confirms the presence of SH group in cysteine molecule.24 When cysteine binds on gold surface, the absorption bands at 1600 and 1390 cm−1, which corresponds to the asymmetric and symmetric stretching of –COOH, and the 3000–3500 cm−1 bands of –NH3+ stretch became weak. This is likely due to a change in their dipole moment when cysteine binds on metal surface with high electron density. Significantly, the S–H stretching band at 2550 cm−1 of cysteine disappeared in the IR spectrum of L-cysteine-modified AuNPs, which suggested the formation of Au–S bond. This is consistent with the results reported in the literature.25 These results demonstrated the successful modification of L-cysteine on the surface of AuNPs.According to the TEM image in Fig. 2a, the as-synthesized AuNPs were spherical and dispersed, and the average size of the AuNPs was 13 nm. The zeta potential is one index of the colloid stability.26 The zeta potential of AuNPs and L-Cys–AuNPs were −36.1 and −25.0 mV, respectively (Fig. S2†). This suggested that the L-Cys–AuNPs solution was enough stable. Furthermore, the stability of L-Cys–AuNPs solution was estimated by measurement of the absorbance at 520 nm every day. The results showed that the absorbance of L-Cys–AuNPs solution remained almost unchanged during two weeks (ESI, Fig. S3†). So, L-Cys–AuNPs showed very good stability in water. It was also found that they remain considerably stable at a wide pH value from 3.0 to 10.0 (Fig. S4†). The above results show that L-Cys–AuNPs solution was enough stable for colorimetric chiral assay.
 | ||
| Fig. 2 TEM images of L-Cys–AuNPs (a) and L-Cys–AuNPs after treatment with D-Dopa (b) and L-Dopa (c). | ||
Colorimetric chiral discrimination of Dopa
In order to study the chiral recognition ability of L-Cys–AuNPs, D-Dopa and L-Dopa were added into the AuNPs solution, respectively. The absorbance spectra and color of the AuNPs solution responding to D-Dopa or L-Dopa was presented in Fig. 3. In the presence of L-Dopa, a well-marked red-to-blue color change can be observed. In the meantime, the absorbance at 520 nm reduced and a new absorbance peak emerged at 650 nm, and the visible spectrum of the system displayed the characteristic red shift and broadening of the surface plasmon band. However, the spectrum shift would not take place in the presence of D-Dopa, and the red-to-blue color change would be not observed. Thus, the changes in solution color and absorption spectra of L-Cys–AuNPs allow one to discriminate D-Dopa and L-Dopa.In order to know the microstructure of L-Cys–AuNPs with D-Dopa and L-Dopa, the TEM images (Fig. 2) were obtained. Note that in the presence of L-Dopa, L-Cys–AuNPs aggregated together, whereas the AgNPs were mono-dispersed in the presence of D-Dopa. These results indicate that L-Dopa can induce aggregation of L-Cys–AuNPs, but D-Dopa did not affect. The results were consistent with the changes in solution color and absorption spectra of L-Cys–AuNPs (Fig. 3).
Optimization of experimental conditions of chiral recognition
In order to improve the performance of chiral recognition, the experimental conditions were optimized. The influence of media pH on the aggregation rate of AuNPs is rather complex, such as dissociation constant, equivalent point and hydrogen bonding interactions.27 Firstly, the effect of media pH on the response of L-Cys–AuNPs was investigated at a pH from 3.0 to 10.0. The experimental results (Fig. S5†) showed that at pH 3.0, both L-Dopa and D-Dopa could induce the aggregation of L-Cys–AuNPs (the solution color became blue), and at pH 6.0 or above 6.0 the color of L-Cys–AuNPs solution was still red in the presence of L-Dopa or D-Dopa. That is to say, under low pH or high pH, L-Cys–AuNPs could not discriminate L-Dopa and D-Dopa. Only at pH 4.0 and 5.0, L-Cys–AuNPs could discriminate L-Dopa and D-Dopa, and the maximum difference between Dopa enantiomers was obtained at pH 4.0. Therefore, media pH 4.0 was chosen in this study. L-Cys–AuNPs amount and incubation time also affected the chiral recognition of Dopa. We investigated the effect of L-Cys–AuNPs amount in the range of 80–160 μL. The experimental results (Fig. S6†) showed that 100 μL L-Cys–AuNPs (17.6 nM) can satisfactorily discriminate 100 μM D- and L-Dopa. Furthermore, after adding enantiomerically pure Dopa, UV-vis spectra of the assay solution were recorded at different incubation time. For L-Dopa, the absorption ration (A650/A520) gradually increased with the incubating time from 2 to 15 min, and after 15 min the absorption ration did not change. But D-Dopa did not induce the aggregation of L-Cys–AuNPs under the same conditions. So, the incubation time was fixed at 15 min.Response of L-Cys–AuNPs to D- and L-Dopa with different concentrations
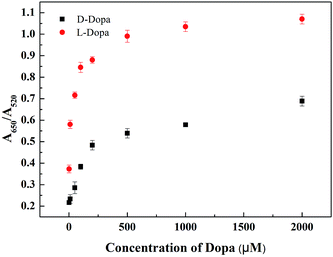
Under the optimized conditions, experiments were carried out by adding increasing amounts of D- or L-Dopa to the L-Cys–AuNPs solution in order to examine whether L-Cys–AuNPs could be used as probe to detect chiral Dopa. The UV-visible spectra of L-Cys–AuNPs in the presence of D- or L-Dopa with different concentrations in the range of 1–2000 μM were recorded. When L-Dopa concentration increased from 1 μM to 2000 μM, the absorbance at 520 nm gradually decreased accompanied with an increase of absorbance at 650 nm (Fig. S7†). In sharp contrast to L-Dopa, there was no distinct effect on the L-Cys–AuNPs upon the addition of D-Dopa, whose concentration was less than 200 μM (Fig. S8†). As shown in Fig. 4, a dramatic increase in the absorption ratio (A650/A520) of L-Cys–AuNPs was observed in the presence of L-Dopa over concentration range from 1 to 100 μM; however, in the same condition, the A650/A520 ratio of L-Cys–AuNPs toward D-Dopa increased less. The data (Fig. 4) indicated that the resulting A650/A520 ratio for 10 μM L-Dopa was equal to that of 1000 μM D-Dopa. This suggests that the aggregation induced by L-Dopa is much more sensitive than by D-Dopa by 2 orders of magnitude. The limit of discrimination concentration between L- and D-Dopa is about approximately 1 μM (Fig. 4) according to the absorption ratio (A650/A520). The clear difference in the results for the two enantiomers suggests that the L-Cys–AuNPs colorimetric probes are capable of discriminating enantiomers of Dopa.Chiral recognition mechanism
The elucidation of chiral recognition mechanism is the key question for chiral recognition and chiral separation. In order to explore the chiral recognition mechanism of the system, we prepared citrate-capped AuNPs and the bare AuNPs through direct reaction of HAuCl4 aqueous solution and NaBH4 aqueous solution (without organic capping regent),28 and the responses of citrate-capped AuNPs, the bare AuNPs and L-Cys-capped AuNPs to L- and D-Dopa were compared. The experimental results showed that the bare AuNPs and citrate-capped AuNPs could not discriminate L- and D-Dopa. This suggests that the chiral recognition ability of L-Cys-capped AuNPs is due to L-Cys adsorbed on the AuNPs surface. The chiral selectivity is attributed to the preferential interaction between chiral selector and one of the enantiomers. To study the interaction between L-Cys-capped AuNPs and Dopa enantiomers, 100 μL L-Cys-capped AuNPs solution was mixed with 50 μL 2 mM L-Dopa or D-Dopa, and after incubation for 15 min the mixture solution was centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. A lot of precipitate could be collected in the bottom of tube. The content of Dopa in the supernatant was monitor with ninhydrin colorimetric method (ninhydrin can react with Dopa to produce blue compound). We compared the absorption value at 570 nm (Fig. S9†) of Dopa solution before and after treatment with L-Cys-capped AuNPs. It was obvious that the content of L-Dopa in the supernatant markedly reduced. However, no obvious changes occurred in the D-Dopa tube under the same condition. On the other hand, the precipitate collected in the bottom of tube was re-dispersed with water, and we measured the CD spectra of the re-dispersed solution. The results (Fig. 5) showed that L-Cys-capped AuNPs/L-Dopa system exhibited a strong positive signal at 220 nm in CD spectra. As the control experiment, L-Cys-capped AuNPs themselves did not exhibit obvious CD signal at 220 nm. And L-Cys-capped AuNPs/D-Dopa system also did not exhibit obvious CD signal at 220 nm. L-type enantiomer often exhibits positive CD signal at 220 nm, and D-type enantiomer always has negative CD signal.13 The CD results suggested that L-Dopa could absorb on L-Cys-capped AuNPs whereas D-Dopa did not adsorb on L-Cys-capped AuNPs. The results agreed with the change of L-Dopa (or D-Dopa) content in the supernatant, and the reduction of L-Dopa in the supernatant was attributed to the fact that L-Cys-capped AuNPs could bind L-Dopa. These experimental results reveal that the interaction of L-Dopa and L-Cys-capped AuNPs is greater than that of D-Dopa and L-Cys-capped AuNPs. This observation is consistent with the work of previous researchers, who demonstrated that the homochiral interaction is usually stronger than heterochiral interactions.29,30 That is to say, L-Dopa exhibits an “ideal fit” with L-Cys-capped AuNPs. Therefore, the selective response may be attributed to the fact that the conformation of L-Dopa is more inclined ro interact with L-Cys-capped AuNPs than D-Dopa. L-Dopa is likely to interact with ligand L-cysteine via carboxylic, amino, and hydroxyl groups through electrostatic interactions and hydrogen bonding resulting in the aggregation of AuNPs. In other words, the homochiral interaction between L-Dopa and L-Cys-capped AuNPs is considerably more favoral than the heterochiral interaction between D-Dopa and L-Cys-capped AuNPs. According to the above proposed mechanism for chiral recognition, we speculated that D-Dopa exhibits an “ideal fit” with D-Cys-capped AuNPs. To validate the scenario, we prepared D-Cys-capped AuNPs, and then L-Dopa and D-Dopa was added into the D-Cys-capped AuNPs solution, respectively. The experimental results (Fig. S10†) showed that a noticeable red-to-blue color change of D-Cys–AuNPs solution can be observed in the presence of D-Dopa, whereas no color change is found in the presence of L-Dopa. Thus, the proposed mechanism for chiral recognition in this system was reliable.
000 rpm for 15 min. A lot of precipitate could be collected in the bottom of tube. The content of Dopa in the supernatant was monitor with ninhydrin colorimetric method (ninhydrin can react with Dopa to produce blue compound). We compared the absorption value at 570 nm (Fig. S9†) of Dopa solution before and after treatment with L-Cys-capped AuNPs. It was obvious that the content of L-Dopa in the supernatant markedly reduced. However, no obvious changes occurred in the D-Dopa tube under the same condition. On the other hand, the precipitate collected in the bottom of tube was re-dispersed with water, and we measured the CD spectra of the re-dispersed solution. The results (Fig. 5) showed that L-Cys-capped AuNPs/L-Dopa system exhibited a strong positive signal at 220 nm in CD spectra. As the control experiment, L-Cys-capped AuNPs themselves did not exhibit obvious CD signal at 220 nm. And L-Cys-capped AuNPs/D-Dopa system also did not exhibit obvious CD signal at 220 nm. L-type enantiomer often exhibits positive CD signal at 220 nm, and D-type enantiomer always has negative CD signal.13 The CD results suggested that L-Dopa could absorb on L-Cys-capped AuNPs whereas D-Dopa did not adsorb on L-Cys-capped AuNPs. The results agreed with the change of L-Dopa (or D-Dopa) content in the supernatant, and the reduction of L-Dopa in the supernatant was attributed to the fact that L-Cys-capped AuNPs could bind L-Dopa. These experimental results reveal that the interaction of L-Dopa and L-Cys-capped AuNPs is greater than that of D-Dopa and L-Cys-capped AuNPs. This observation is consistent with the work of previous researchers, who demonstrated that the homochiral interaction is usually stronger than heterochiral interactions.29,30 That is to say, L-Dopa exhibits an “ideal fit” with L-Cys-capped AuNPs. Therefore, the selective response may be attributed to the fact that the conformation of L-Dopa is more inclined ro interact with L-Cys-capped AuNPs than D-Dopa. L-Dopa is likely to interact with ligand L-cysteine via carboxylic, amino, and hydroxyl groups through electrostatic interactions and hydrogen bonding resulting in the aggregation of AuNPs. In other words, the homochiral interaction between L-Dopa and L-Cys-capped AuNPs is considerably more favoral than the heterochiral interaction between D-Dopa and L-Cys-capped AuNPs. According to the above proposed mechanism for chiral recognition, we speculated that D-Dopa exhibits an “ideal fit” with D-Cys-capped AuNPs. To validate the scenario, we prepared D-Cys-capped AuNPs, and then L-Dopa and D-Dopa was added into the D-Cys-capped AuNPs solution, respectively. The experimental results (Fig. S10†) showed that a noticeable red-to-blue color change of D-Cys–AuNPs solution can be observed in the presence of D-Dopa, whereas no color change is found in the presence of L-Dopa. Thus, the proposed mechanism for chiral recognition in this system was reliable.
 | ||
| Fig. 5 CD spectra of L-Cys–AuNPs after treatment with D-Dopa or L-Dopa. Experiment conditions: 100 μL L-Cys–AuNPs, 300 μL B–R (pH 4.0), 50 μL Dopa (2 mM). | ||
Conclusions
The rational design and construction of optical active ligand capped nanoparticles for chiral recognition is of intense current interest. In this work, we assembled L-cysteine on the surface of AuNPs to construct the chiral AuNPs, and the chiral L-Cys-capped AuNPs were used enantioselective recognition of Dopa enantiomers by the naked eye due to L-Dopa-induced aggregation of L-Cys-capped AuNPs. This method can achieve the goal of translating an enantioselective molecular recognition event into an appreciable color change. The assay described in this work is easily readout with the naked eye or using a UV-vis spectrometer. Compared with the common method for chiral recognition such as HPLC, GC, CD and NMR, this method is more attractive because of its sensitivity, low cost, ready availability and simple manipulation. Furthermore, through simple substitution of other optical active ligand for L-cysteine, the methodology can be extended to detect other chiral compounds.Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (no. 21275096 and 21475083), Shaanxi Provincial Natural Science Foundation (no. 2013SZS08-Z01) and Program for Innovative Research Team in Shaanxi Province (no. 2014KCT-28).References
- G. C. Cotzias, P. Papavasi and R. Gellene, N. Engl. J. Med., 1969, 280, 337–341 CrossRef CAS PubMed.
- D. Poskanze, N. Engl. J. Med., 1969, 280, 382–383 CrossRef PubMed.
- J. Moses, A. Siddiqui and P. B. Silverman, Neurosci. Lett., 1996, 218, 145–148 CrossRef CAS.
- M. Wu, X. J. Zhou, R. Konno and Y. X. Wang, Clin. Exp. Pharmacol. Physiol., 2006, 33, 1042–1046 CrossRef CAS PubMed.
- G. Grigorean and C. B. Lebrilla, Anal. Chem., 2001, 73, 1684–1691 CrossRef CAS.
- B. Yuan, H. Wu, T. Sanders, C. McCullum, Y. Zheng, P. B. Tchounwou and Y. M. Liu, Anal. Biochem., 2011, 416, 191–195 CrossRef CAS PubMed.
- C. Borst and U. Holzgrabe, J. Chromatogr. A, 2008, 1204, 191–196 CrossRef CAS PubMed.
- X. Li, D. Xiao, X.-M. Ou, C. McCullm and Y.-M. Liu, J. Chromatogr. A, 2013, 251–256 CrossRef PubMed.
- Q. Han, Q. Chen, Y. Wang, J. Zhou and Y. Fu, Electroanalysis, 2012, 24, 332–337 CrossRef CAS.
- Y. Chen, Y. Huang, D. Guo, C. Chen, Q. Wang and Y. Fu, J. Solid State Electrochem., 2014, 18, 3463–3469 CrossRef CAS.
- L. Chen, F. Chang, L. Meng, M. Li and Z. Zhu, Analyst, 2014, 139, 2243–2248 RSC.
- Y. Huang, Q. Han, Q. Zhang, L. Guo, D. Guo and Y. Fu, Electrochim. Acta, 2013, 113, 564–569 CrossRef CAS PubMed.
- Y. J. Kang, J. W. Oh, Y. R. Kim, J. S. Kim and H. Kim, Chem. Commun., 2010, 46, 5665–5667 RSC.
- T. Tu, W. Fang, X. Bao, X. Li and K. H. Dötz, Angew. Chem., Int. Ed., 2011, 50, 6601–6605 CrossRef CAS PubMed.
- X. Zhang, J. Yin and J. Yoon, Chem. Rev., 2014, 114, 4918–4959 CrossRef CAS PubMed.
- Y. Song, W. Wei and X. Qu, Adv. Mater., 2011, 23, 4215–4236 CrossRef CAS PubMed.
- W. Zhao, M. A. Brook and Y. Li, ChemBioChem, 2008, 9, 2363–2371 CrossRef CAS PubMed.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- S. H. Seo, S. Kim and M. S. Han, Anal. Methods, 2014, 6, 73–76 RSC.
- H. Su, Q. Zheng and H. Li, J. Mater. Chem., 2012, 22, 6546–6548 RSC.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735–743 CrossRef CAS.
- R. C. Jin, G. S. Wu, Z. Li, C. A. Mirkin and G. C. Schatz, J. Am. Chem. Soc., 2003, 125, 1643–1654 CrossRef CAS PubMed.
- L. Zhang, C. Xu, C. Liu and B. Li, Anal. Chim. Acta, 2014, 809, 123–127 CrossRef CAS PubMed.
- L. Zhang, C. Xu and B. Li, Microchim. Acta, 2009, 166, 61–68 CrossRef CAS.
- S. Aryal, B. K. C. Remant, N. Dharmaraj, N. Bhattarai, C. H. Kim and H. Y. Kim, Spectrochim. Acta, Part A, 2006, 63, 160–163 CrossRef PubMed.
- T. Kim, K. Lee, M.-S. Gong and S.-W. Joo, Langmuir, 2005, 21, 9524–9528 CrossRef CAS PubMed.
- H. M. Zakaria, A. Shah, M. Konieczny, J. A. Hoffmann, A. J. Nijdam and M. E. Reeves, Langmuir, 2013, 29, 7661–7673 CrossRef CAS PubMed.
- W. Chen, H.-H. Deng, L. Hong, Z.-Q. Wu, S. Wang, A.-L. Liu, X.-H. Lin and X.-H. Xia, Analyst, 2012, 137, 5382–5386 RSC.
- M. Matsunaga, T. Nakanishi, T. Asahi and T. Osaka, Chirality, 2007, 19, 295–299 CrossRef CAS PubMed.
- T. Nakanishi, M. Matsunaga, M. Nagasaka, T. Asahi and T. Osaka, J. Am. Chem. Soc., 2006, 128, 13322–13323 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01271f |
| This journal is © The Royal Society of Chemistry 2015 |