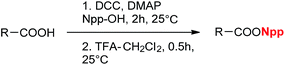2-(2-Nitrophenyl) propyl: a rapidly released photolabile COOH-protecting group for solid-phase peptide synthesis†
Gang Wangab,
Tao Pengb,
Shouguo Zhangb,
Junyi Wangc,
Xiaoxue Wenb,
Haiyan Yanb,
Liming Hu*a and
Lin Wang*ab
aCollege of Life Science and Bio-engineering, Beijing University of Technology, Beijing, 100124, P. R. China.. E-mail: wanglin@bmi.ac.cn; huliming@bjut.edu.cn
bBeijing Institute of Radiation Medicine, Beijing, 100850, P. R. China
cSchool of Basic Medical Sciences, Peking University, Beijing, 100191, P. R. China
First published on 13th March 2015
Abstract
We developed a new efficient photolabile protecting group, 2-(2-nitrophenyl) propyl (Npp) that blocks the carboxyl group in peptide synthesis. The Npp group is quite compatible with the Fmoc–t-Bu strategy and can be removed rapidly and quantitatively by irradiation with UV light. Using this method, we prepared a head-to-tail cycloheptapeptide.
An increasing number of peptides have been obtained from nature, and their properties of high bioactivity and low toxicity have attracted interest from pharmaceutical researchers. However, as a result of proteolytic enzymes, these peptides have a critical drawback that limits their stability in biological fluids.1 In many situations, the flexibility of linear peptides confers a labile conformation, whereas the head-to-tail cyclization of a linear peptide via amide bond formation can constrain the conformation of a peptide or protein, increasing its stability against proteolysis. Furthermore, head-to-tail cyclization can increase the binding affinity by favoring entropic molecular recognition. Because cyclic peptides can exhibit enhanced resistance to proteolysis and thus exhibit improved pharmacokinetic profiles, the head-to-tail cyclic peptide strategy has been adopted by pharmaceutical chemists to prepare natural products with increased biostability and bioactivity.
An increasing number of head-to-tail peptide compounds have been formed from naturally obtained resources.2 Various solution-phase3 and solid-phase4 methods have been reported for the synthesis of head-to-tail cyclic peptides. In the solution phase, the cyclization must be conducted under high dilution conditions. In the solid phase, the peptide is synthesized on a solid support and cyclized while the molecule is still attached to the resin. The synthesis of cyclic peptides in the solid phase benefits from the numerous advantages of the “pseudo-dilution” principle. The solid-phase method is an excellent approach that avoids tedious purification steps after each individual chemical step and diminishes the side reactions and polymerization of molecules, thus facilitating purification. In this strategy, the starting amino acid residue is anchored to the support via its side chain on the basis of the three-dimensional orthogonal scheme of protection. This approach demands that the head-to-tail cyclopeptide contain at least one amino acid with an active side chain, such as a hydroxy (Thr, Ser, Tyr), amino (Lys, Orn), or carboxyl (Asp, Glu) group. This method can free the N- and C-termini from the resin so they can join each other and complete the required cyclization in the solid phase.
The side-chain anchoring strategy generally involves the side chain of an Fmoc-AA-allyl peptide and the deprotection of the allyl ester by a Pd reagent, although this approach is quite expensive. The solvent used for deprotection always requires special treatment, such as extreme drying and hypoxic conditions. Another drawback is that the removal of the allyl group makes monitoring of the end point difficult. These shortcomings demand the development of a new and widely compatible protecting group that can overcome these drawbacks. Thus, we have turned our research direction to developing a strategy that involves a mild and effective photolabile protecting group.
Photo-removable protecting groups play an important role in a variety of syntheses. Barltrop et al.5 first reported the use of an o-nitrobenzyl group to release benzoic acid. Gee et al.6 employed 2,2′-dinitrobenzhydryl (DNB) as a modification to an o-nitrobenzyl group to block the carboxyl group of N-methyl-D-aspartate. Benzoin esters7 have also been developed into an efficient method to block the carboxyl group. Many photolabile protecting groups and linkers have been reported; however, the range of their applications in solid-phase peptide synthesis (SPPS) is limited. An important factor is the low efficiency of photolysis. The solid support reduces light transparency; therefore, the removal of the group inside the bead is not as efficient as the removal of groups on the surface. On the basis of this consideration, if the photolabile group used for SPPS can be rapidly and completely released under light irradiation, the group should exhibit high quantum efficiency and be highly light-sensitive. The photo-product should also be inert and capable of being safely removed without any unwanted side reactions. The other factors involved in the stability of the peptide synthesis reaction are mild chemical conditions and the presence of active groups such as –NH2, –OH, –SH, and –COOH in the amino acids.
In the late 1990s, Pfleiderer and co-workers8 developed 2-(o-nitrophenyl) alkyloxycarbonate, a new β-substituted variant of the o-nitrobenzyl group. The photo-release of this group is believed to occur through a β-elimination mechanism9 which differs from the release of an o-nitrobenzyl photolabile group. Bhushan et al.10 used the 2-(2-nitrophenyl) propyloxy carbonyl group (Nppoc) as a protecting group to block only the amino group in the amino acid. A comparison with the corresponding 6-nitroveratroyloxycarbonyl group (Nvoc) amino acids in solution under identical conditions indicated that cleavage of the Nppoc derivatives occurred approximately twice as fast as cleavage of the corresponding Nvoc derivatives.11 These results indicate that these types of protecting groups are of considerable interest in both small-molecule synthesis and peptide synthesis, which has not been previously reported in literature. On the basis of these considerations, we considered the 2-(2-nitrophenyl) propyl (Npp) group to be an excellent photolabile protecting candidate for use in cyclic peptide synthesis.
To obtain the Npp-protected acid, 2-(2-nitrophenyl) propanol (Npp–OH) (2) was synthesized via the hydroxymethylation of o-nitroethylbenzene with paraformaldehyde in the presence of the base Triton B (Scheme 1), which facilitated the reaction and provided high product yields. The corresponding Npp ester was easily prepared through a routine esterification procedure (Table 1). The yields were satisfactory, and the purification process was simple. The structures of the products were assigned by 1H NMR, 13C NMR and HR-MS.
| a The total yield was calculated for two steps involving esterification and t-Bu cleavage. | ||
|---|---|---|
 3a 83.2% 3a 83.2% |
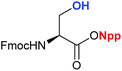 3b 96.9% 3b 96.9% |
 3c 92.4% 3c 92.4% |
 3d 98.2% 3d 98.2% |
 3e 77.4% 3e 77.4% |
 3f 89.3% 3f 89.3% |
 3g 90.1% 3g 90.1% |
 3h 90.1% 3h 90.1% |
|
To test the compatibility of the Npp ester with other protecting groups, CH3COONpp was synthesized as a model compound and was dissolved in 20% piperidine-DMF solution and TFA solution to ensure compatibility with t-Bu and Fmoc, respectively. The solutions were left standing in the dark for 48 h at room temperature. No obviously visible change of either solvent was observed by TLC monitoring, except for a slight color change of the piperidine solution to light-yellow. This result implies that the active side chain containing the Npp ester can be exposed for solid-phase synthesis under acidolysis-inducing conditions and in piperidine solution.
The photolysis-mediated release of the Npp ester was UV-induced with constant Ar bubbling at a rapid rate in a wide range of solvents, including THF, CH3CN, MeOH and DMF. Among the several solvents used in these preliminary photo-deprotection studies, DMF provided the best results. In DMF, the deprotection of Npp under high pressure Hg lamp (λmax = 365 nm, 250 W, power density = 2.95 W cm−2, irradiation area was calculated to be 4.9 cm2) was completed in 30 min. The addition of a solvent with a low boiling point, such as MeOH, was necessary to decrease the effects of heat produced by the UV light. Commercially available solvent can be used directly in photolysis without any special treatment except pumping for 5 to 10 min to remove dissolved oxygen.
To facilitate photocleavage, the photolysis performed in the quartz tube was the most effective method. However, the photodeprotection of Npp can also perform smoothly in the ordinary glass tube, but it is not as rapid as in the quartz tube. It can be removed in 30 min in the quartz tube, but in the glass tube, it always needs 60 min at least.
Another advantage of using the Npp group is that the photolysis products are the substituted o-nitrostyrene and N-hydroxyoxindol,9a which are easily detectable and non-reactive. By contrast, the photo-degradation products of the o-nitrobenzyl group, which include nitrous and carbonyl compounds, may react with the amino groups in amino acids, resulting in reduced stepwise synthetic yields. Moreover, the o-nitrostyrene and N-hydroxyoxindol were dark-yellow in color, which made determination of the end of the reaction straightforward via observation of the color change of the solvent (Scheme 2).
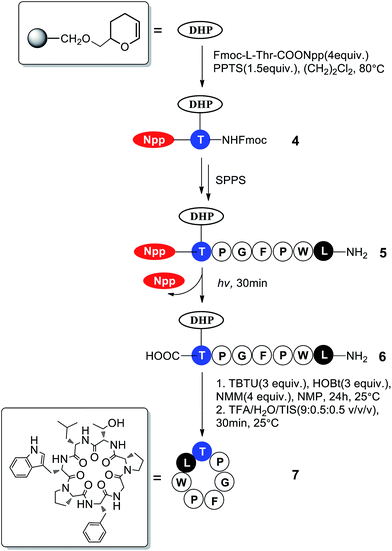
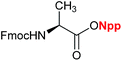
Phakellistatin 13,2b a cyclopeptide containing a Thr residue, was chosen to be synthesized to demonstrate the use of the Npp strategy in peptide synthesis (Scheme 3). The Fmoc- and Npp-protected Thr residue was used as the starting material. The side hydroxy of Thr was anchored to the DHP resin using the Elleman method,12 where the linker can be split in a weakly acidic solvent such as 10% TFA–CH2Cl2. The deprotection of the N-terminus of Fmoc was performed using 20% piperidine in DMF, and the formation of the peptide bond was accomplished using TBTU as a coupling agent in the presence of HOBt and 4-methylmorpholine NMM in DMF. Each amino-acid coupling step was monitored by the ninhydrin test. During the SPPS, the formation of diketopiperazine, the product of an undesired side reaction that occurs at the dipeptide level during the removal of Fmoc in piperidine–DMF solution (especially when the Pro residue is in the second position), did not occur.
After the solid-state synthesis of the linear heptapeptide was completed, the Fmoc group protecting the Leu residue was removed. The Npp group at the C-terminus was subsequently removed by irradiation with a high pressure Hg lamp (λmax = 365 nm, 250 W, power density = 2.95 W cm−2, irradiation area was calculated to be 4.9 cm2) twice for 15 min in DMF–MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
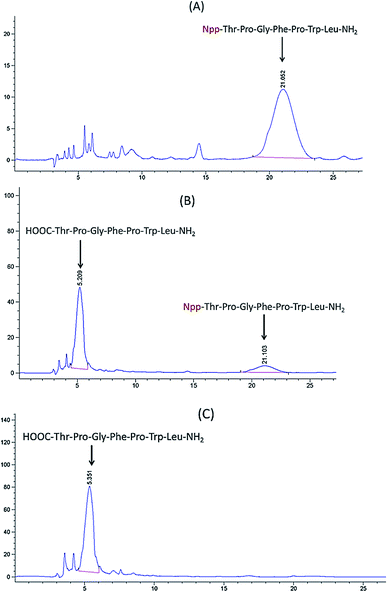
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) until the color remained unchanged, indicating the complete removal of Npp. To confirm this result, HPLC experiments were performed after cleavage of the microscale peptide resin (Fig. 1); the HPLC results indicated the complete cleavage of Npp and thus verified the authenticity of Npp as a photolabile protecting group. Then cyclization reaction was then allowed to proceed in the solid phase with TBTU–NMM activation in NMP. The peptide was cut from the resin, and the crude cyclic peptide was purified by flash column chromatography (CH2Cl2
1, v/v) until the color remained unchanged, indicating the complete removal of Npp. To confirm this result, HPLC experiments were performed after cleavage of the microscale peptide resin (Fig. 1); the HPLC results indicated the complete cleavage of Npp and thus verified the authenticity of Npp as a photolabile protecting group. Then cyclization reaction was then allowed to proceed in the solid phase with TBTU–NMM activation in NMP. The peptide was cut from the resin, and the crude cyclic peptide was purified by flash column chromatography (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 9
MeOH = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Finally, the product was analysis by HPLC at 220 nm with 70% CH3OH–H2O (0.1% TFA) as eluent system (Fig. 2) and characterized by ESI-TOF-MS.
1). Finally, the product was analysis by HPLC at 220 nm with 70% CH3OH–H2O (0.1% TFA) as eluent system (Fig. 2) and characterized by ESI-TOF-MS.
In summary, we report the use of a rapid, effective photolabile protecting group for carboxyl groups in SPPS. Meanwhile, we provide a new method for head-to-tail cyclic peptide synthesis. The Npp group is tolerated in the reaction conditions used to cleave the t-Bu ester and the Fmoc group. Npp is also rapidly removed by UV irradiation, without the occurrence of any unwanted side reactions. The present work provides a new tool for the synthesis of complicated peptides.
Abbreviations
| Boc | tert-Butyloxycarbonyl |
| Cbz | Benzyloxycarbonyl |
| DCC | Dicyclohexylcarbodiimide |
| DCM | Dichloromethane |
| DMAP | 4-(N,N-Dimethylamino)pyridine |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethylsulfoxide |
| ESI-TOF | Electrospray ionization-time of flight technique |
| NMP | N-Methyl-2-pyrrolidone |
| EtOAc | Ethyl acetate |
| Et2O | Diethyl ether |
| Fmoc | 9-Fluorenylmethoxycarbonyl |
| NMM | 4-Methylmorpholine |
| Npp | 2-(2-Nitrophenyl)propyl |
| HOBt | 1-Hydroxybenzotriazole |
| HPLC | High-pressure liquid chromatography |
| NMR | Nuclear magnetic resonance |
| PPTS | Pyridinium p-toluenesulfonate |
| TIS | Tris-isopropylsilane |
| TBTU | O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate |
| t-Bu | tert-Butyl |
| TFA | Trifluoroacetic acid |
Acknowledgements
We are thankful to the National Science Foundation of China for providing financial support (Grant no. 81273431, 81072531, 30770655 and 21102176).Notes and references
- D. P. McGregor, Curr. Opin. Pharmacol., 2008, 8, 616 CrossRef CAS PubMed.
- (a) A. Napolitano, I. Bruno, P. Rovero, R. Lucas, M. P. Peris, L. Gomez-Paloma and R. Riccio, Tetrahedron, 2001, 57, 6249 CrossRef CAS; (b) A. Wele, Y. J. Zhang, L. Dubost, J. L. Pousset and B. Bodo, Chem. Pharm. Bull., 2006, 54, 690 CrossRef CAS; (c) F. R. Chang, J. L. Chen, H. F. Chiu, M. J. Wu and Y. C. Wu, Phytochemistry, 1998, 47, 1057 CrossRef CAS; (d) G. R. Pettit, J. K. Srirangam, D. L. Herald, K. L. Erickson, D. L. Doubek, J. M. Schmidt, L. P. Tackett and G. J. Bakus, J. Org. Chem., 1992, 57, 7217 CrossRef CAS.
- (a) G. R. Pettit, B. E. Toki, J. P. Xu and D. C. Brune, J. Nat. Prod., 2000, 63, 22 CrossRef CAS PubMed; (b) A. Amore, R. Van Heerbeek, N. Zeep, J. Van Esch, J. N. H. Reek, H. Hiemstra and J. H. Van Maarseeen, J. Org. Chem., 2006, 71, 1851 CrossRef CAS PubMed; (c) J. N. Lambert, J. P. Mitchell and K. D. Roberts, J. Chem. Soc., Perkin Trans. 1, 2001, 471 RSC.
- (a) K. L. Greenman, D. M. Hach and D. L. Van Vranken, Org. Lett., 2004, 6, 1713 CrossRef CAS PubMed; (b) G. T. Bourne, S. W. Golding, R. P. McGeary, W. D. F. Meutermans, A. Jones, G. R. Marshall, P. F. Alewood and M. L. Smythe, J. Org. Chem., 2001, 66, 7706 CrossRef CAS PubMed; (c) N. Tamilarasu, I. Huq and T. M. Rana, Bioorg. Med. Chem. Lett., 2000, 10, 971 CrossRef CAS.
- J. A. Barltrop, P. J. Plant and P. Schofield, J. Chem. Soc., Chem. Commun., 1966, 822 RSC.
- K. R. Gee, L. Niu, K. Schaper, V. Jayaraman and G. P. Hess, Biochemistry, 1999, 38, 3140 CrossRef CAS PubMed.
- (a) J. C. Sheehan and R. M. Wilson, J. Am. Chem. Soc., 1964, 86, 5277 CrossRef CAS; (b) J. C. Sheehan, R. M. Wilson and A. W. Oxford, J. Am. Chem. Soc., 1971, 93, 7222 CrossRef CAS.
- A. Hasan, K. P. Stengele, H. Giegrich, P. Cornwell, K. R. Isham, R. A. Sachleben, W. Pfleiderer and R. S. Foote, Tetrahedron, 1997, 53, 4247 CrossRef CAS.
- (a) H. Giegrich, S. Eisele-Buhler, C. Hermann, E. Kvasyuk, R. Charubala and W. Pfleiderer, Nucleosides Nucleotides, 1998, 17, 1987 CrossRef CAS; (b) S. Walbert, W. Pfleiderer and U. E. Steiner, Helv. Chim. Acta, 2001, 84, 1601 CrossRef CAS.
- K. R. Bhushan, C. DeLisi and R. A. Laursen, Tetrahedron Lett., 2003, 44, 8585 CrossRef CAS PubMed.
- (a) M. Beier and J. D. Hoheisel, Nucleic Acids Res., 2000, 28, e11 CrossRef CAS PubMed; (b) M. Beier and J. D. Hoheisel, Nucleic Acids Res., 1999, 27, 1970 CrossRef CAS PubMed.
- L. A. Thompson and L. A. Ellman, Tetrahedron Lett., 1994, 35, 9333 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterizations, and spectroscopic data are available. See DOI: 10.1039/c5ra01210d |
| This journal is © The Royal Society of Chemistry 2015 |