Structural diversity of terpenoids in the soft coral Sinularia flexibilis, evidenced by a collection from the South China Sea
Wen-Ting Chen,
Jia Li,
Jian-Rong Wang,
Xu-Wen Li* and
Yue-Wei Guo*
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Zhang Jiang High-Tech Park, Shanghai, 201203, People's Republic of China. E-mail: ywguo@simm.ac.cn; xuwen.li@org.chem.ethz.ch; Fax: +86 21 50805813; Tel: +86 21 50805813
First published on 25th February 2015
Abstract
Eight new terpenoids, including six α-methylene-δ-lactone-bearing cembranoids (1–6), a 15-membered macrocyclic diterpenoid (7), and a biscembranoid (8), were isolated from South China Sea soft coral Sinularia flexibilis, along with five known analogues (9–13). Their structures including relative stereochemistry were elucidated by detailed spectroscopic analyses, chemical reactions and by comparison with literature data. Further, the structures of 8 and 10 were unambiguously confirmed by X-ray diffraction analyses. Compound 7, named epoxyflexibilene, represents the second 15-membered macrocyclic diterpenoid being discovered from marine sources, whereas sinulaflexiolide L (8) is the third member of the extremely rare cembrane dimers connected through C–C single bond. The discovery of these new isolates showed the high chemical diversity and ecological complexity of the animal S. flexibilis collected in different locations. In a bioassay, compound 9 exhibited potent anti-tumor activity targeting the inositol-requiring 1/X-box-binding protein 1 (IRE1/XBP1) signaling pathway.
Introduction
Soft corals of the genus Sinularia (phylum Cnidaria, class Anthozoa, subclass Octocorallia, order Alcyonacea, family Alcyoniidae) are prolific in the South China Sea, which have been well recognized as a rich source of bioactive substances with intriguing structural features, including rearranged terpenoids and biscembranoids.1 The genus Sinularia consists of approximately 90 species, of which more than 50 have been chemically investigated. S. flexibilis, standing as a popular species of them, has long been attractive thanks to its highly diverse constitutions. Many terpenoids with broad structural variations have been isolated in S. flexibilis collected from Taiwan,2–5 North Queensland,6 Philippine,7 Hainan,8 etc. It has been suggested that such soft coral species in different habitat comprise different secondary metabolites. For instance, furanoditerpenes are isolated from this species collected from Philippine,6 while a structurally unique sulfur-contained biscembranoid, namely thioflexibilolide A, was only isolated from an Taiwan soft coral S. flexibilis.5 The diverse structure features are probably related to the defensive system of the soft coral against their enemies in various marine ecological environment,9 and these diverse secondary metabolites were highly biologically focused on, which were found to be significant bioactive, such as cytotoxic,2,10 antimicrobial,11 and anti-inflammatory.12 Therefore, S. flexibilis is always attractive for its chemical and biological investigation based on different collected locations. With this aim, and in the course of our continuing research on bioactive secondary metabolites from the South China Sea soft corals,13–17 we collected the specimen S. flexibilis off Yalong Bay, Hainan Island. Chemical investigation of Et2O-soluble fraction of the acetone extract of the title animal led to the isolation of six new α-methylene-δ-lactone-bearing cembranoids, 9α-hydroxy-flexibilide (1), 15(17)-dehydromanaarenolide E (2), 8-dehydroxy-15(17)-dehydromanaarenolide E (3), 15(17)-dehydromanaarenolide A (4), 15(17)-dehydromanaarenolide C (5), epi-flexilarin A (6), a new 15-membered ring macrocyclic diterpenoid epoxyflexibilene (7), and a new biscembranoid sinulaflexiolide L (8), together with five known related cembrane derivatives (9–13) (Fig. 1). Among them, epoxyflexibilene (7) represents the second 15-membered macrocyclic diterpenoid being discovered from marine sources,6 and sinulaflexiolide L (8) is the third member of the rare cembrane dimers connected through C–C single bond.3,8 This paper addresses the isolation, structure elucidation of these compounds and their bioassay results.Results and discussion
Specimens of S. flexibilis were immediately frozen to −20 °C and stored at that temperature before they were exhaustively extracted by acetone. The Et2O-soluble portion of the extract was subjected to repeated column chromatography (silica gel, Sephadex LH-20, and reversed-phase HPLC) to yield 13 cembrane derivatives (1–13). The known compounds were identified as flexibilide (9),6 sinuflexolide (10),2 11-acetylsinuflexolide (11),18 capillolide (12),19 and sinulaflexiolide A (13)8 by analysis of their NMR spectroscopic data, along with comparison with those reported.Sinuflexolide (10) and 11-acetylsinuflexolide (11), exhibiting significant2 and moderate18 cytotoxicity against the growth of human tumor cell lines, respectively, have only been reported for their relative configurations.2,18 With the aim to determine the absolute configurations of both 10 and 11, a stereochemical study was conducted by using X-ray diffraction analysis and chemical reaction conversion. X-ray diffraction experiments with Cu-Kα (λ = 1.54178 Å) radiation allowed the assignment of the absolute configurations to 10 as 1R, 3R, 4S, 11S, 12R. The chemical conversion of 10 into 11 by simple acetylation in Ac2O/pyridine indicated the absolute configuration of 11 is the same as 10, which is also accordant with that of 9.6
Compounds 1–6 showed IR absorptions indicative of the presence of hydroxyl groups (3360–3460 cm−1) and α-methylene-δ-lactone moieties (1718–1731, 1611–1619 cm−1). Their NMR spectra were reminiscent of those of flexibilide (9), which is most frequently isolated in many soft corals belong to Sinularia. In fact, compounds 1–6 displayed the same structural unit extending from C-5 to C-1, C-9 to C-14 and C-1 to C-17, including two affiliated methyl at C-4 and C-12, an α,β-unsaturated-δ-lactone moiety, an epoxy group, and two oxygenated quaternary carbon atoms. The differences consist in either oxidative patterns, or isomerization and migration of double bonds.
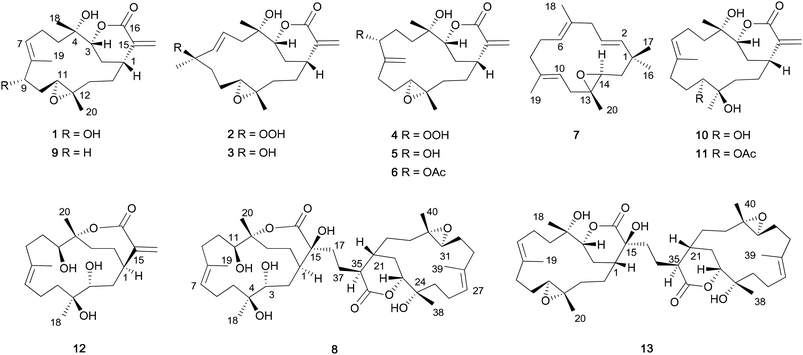
9α-Hydroxy-flexibilide (1) was obtained as an optically active colorless oil. HRESIMS, 13C NMR and DEPT spectra established the molecular formula as C20H30O5. A comparison of the NMR data of 1 with those of 9 revealed a close similarity with the exception of a methylene (δH 2.16; δC 35.8) in 9 replaced by a hydroxyl-bearing methine (δH 4.09; δC 75.3, CH) in 1, which is in agreement with a plus of sixteen mass units in 1. The location of the hydroxyl group was assigned at C-9 by a series of obvious proton connections of H-9/H-10/H-11 as established by a 1H-1H COSY experiment and HMBC correlations from H-9 (δH 4.09) to C-7, C-8, C-10, C-11 and C-19, respectively (Fig. 2). Due to the presence of 9-OH, 13C NMR data from C-7 to C-10 were downfield shifted, while that of C-11 was upfield shifted with respect to those of 9.
The relative configuration of 1 was determined to be the same as that of 9 by ROESY experiment (Fig. 2). The correlations of H-3 (δH 3.97)/H-7 (δH 5.53), H-7/H-9 (δH 4.09), H-9/H-1 (δH 2.51), H-1/H-3 suggested that H-3, H-7, H-9 and H-1, are all co-facial, which could be arbitrarily assigned to be β-oriented. The cross-peaks of H-3/H3-18 (δH 1.45), and H-1/H3-20 (δH 1.36) were indicative of spatial proximity among these protons, implying that H3-18 and H3-20 were oriented in β-face Thus, the structure of compound 4 was identified as 9α-hydroxy-flexibilide.
On the basis of biogenetic considerations, the absolute configurations at C-1, C-3, C-4, C-11 and C-12 were tentatively suggested as R, R, S, S, and S, respectively. In consequence, the absolute configuration at C-9 was assigned as S.
The structure of compounds 2–6 were determined mainly by comparison of NMR, MS and optical rotation data with those of the known α-methylene-δ-lactone-bearing cembranoids, manaarenolide A, C, and E, previously reported from the same genus of Taiwan soft corals.20
15(17)-Dehydromanaarenolide E (2), a white amorphous powder with a molecular formula of C20H30O6 determined by HRESIMS (m/z 389.1927 [M + Na]+), has two mass units less than that of manaarenolide E. In fact, the only difference was at C-15 position, the linkage of a methyl group (δH 1.36; δC 16.2) in manaarenolide E was replaced by a terminal double bond (δH 6.58 and 5.70; δC 127.4) in 2. The configuration of 2 was determined as (1R, 3R, 4S, 8R, 11S, 12S) by comparison of the NMR data with manaarenolide E (1R, 3R, 4S, 8R, 11S, 12S, 15S) and manaarenolide F (1R, 3R, 4S, 8S, 11S, 12S, 15S), which is also in accordance with the configuration of the related compounds 9 and 10 as discussed above, suggesting that 2 was the 15(17)-dehydro derivative of manaarenolide E.
The molecular weight of 3 [HRESIMS peak at m/z 373.2000 (M + Na)+, C20H30O5], 8-dehydroxy-15(17)-dehydromanaarenolide E, was sixteen mass units less than that of 2. Careful comparison of 1H and 13C NMR data of both compounds (Tables 1 and 2) revealed that the only difference was the presence of a hydroxyl group at C-8 in 3 instead of a hydroperoxyl group in 2. 1H-1H COSY, HSQC, and HMBC experiments allowed the unambiguous definition of the structure of 3. Due to the reduction at 8-OOH, the chemical shift of C-8 was slightly downfield shifted from δC 73.3 to 83.9, which was further supported by chemical transformation. A reduction of 2 with triphenylphosphine yielded 3, as an identical NMR spectroscopic data of the isolated product were observed. On the basis of above evidence, the structure of 3 was determined as the 8-dehydroxy derivative of 15(17)-dehydromanaarenolide E (2).
| Position | 1 | 2 | 3 | 4 | 5 | 7 |
|---|---|---|---|---|---|---|
| a Bruker-DRX spectrometer (500 MHz for compounds 1, 3 and 7, 400 MHz for compounds 2, 4 and 5) in CDCl3, chemical shifts (ppm) referred to CHCl3 (δH 7.26) residual signal. Proton coupling constants (J) in Hz are given in parentheses.b Exchangeable. | ||||||
| 1 | 2.51, m | 2.35, m | 2.38, m | 2.56, m | 2.60, m | |
| 2 | 2.02, dd (14.0, 6.5) | 2.02, m | 2.02, m | 2.02, m | 2.02, m | 5.40, d (15.8) |
| 1.39, m | 1.37, m | 1.38, m | 1.37, m | 1.38, m | ||
| 3 | 3.97, d (10.6) | 4.03, d (11.1) | 4.07, dd (11.1, 1.2) | 3.95, d (10.7) | 3.97, d (10.9) | 5.46, dt (15.8, 6.3) |
| 4 | 2.62, d (6.5) | |||||
| 5 | 1.88, m | 2.51, d (15.6) | 2.50, dt (15.4, 2.6) | 1.68, m | 1.67, m | |
| 1.63, m | 2.38, dd (15.6, 10.5) | 2.44, dd (15.4, 10.2) | 1.58, m | 1.58, m | ||
| 6 | 2.49, m | 5.48, ddd (16.2, 10.5, 2.5) | 5.56, ddd (15.3, 10.2, 2.6) | 2.08, m | 1.89, m | 5.03, t (6.9) |
| 1.88, m | 1.34, m | 1.33, m | ||||
| 7 | 5.53, t (8.1) | 5.62, dd (16.2, 2.5) | 5.71, dd (15.3, 2.6) | 4.50, brs | 4.32, brs | 2.07, m |
| 1.95, m | ||||||
| 8 | 2.13, m | |||||
| 1.92, m | ||||||
| 9 | 4.09, dd (11.1, 4.1) | 2.18, m | 1.85, m | 2.43, m | 2.30, m | |
| 1.67, m | 2.11, m | 2.19, m | ||||
| 10 | 2.27, ddd (14.2, 11.2, 3.3) | 1.84, m | 1.86, m | 1.77, m | 1.93, m | 5.01, t (6.9) |
| 1.72, m | 1.27, m | 1.29, m | 1.70, m | 1.65, m | ||
| 11 | 2.50, m | 2.86, d (7.3) | 2.85, d (9.7) | 2.93, dd (7.1, 4.0) | 2.95, dd (8.9, 3.0) | 2.14, m |
| 12 | 1.92, m | |||||
| 1.51, m | ||||||
| 13 | 2.08, dt (14.1, 3.8) | 2.08, m | 2.13, m | 2.09, m | 2.08, m | |
| 1.16, dt (14.1, 3.8) | 1.23, m | 1.20, m | 1.28, m | 1.29, m | ||
| 14 | 1.91, m | 1.83, m | 2.20, m | 1.90, m | 1.90, m | 2.79, dd (7.6, 3.2) |
| 1.50, m | 1.25, m | 1.48, m | 1.26, m | 1.27, m | ||
| 15 | 1.71, dd (14.3, 3.2) | |||||
| 1.35, dd (14.3, 7.6) | ||||||
| 16 | 1.10, sb | |||||
| 17 | 6.47, d (2.3) | 6.58, d (2.5) | 6.58, d (2.5) | 6.50, s | 6.48, d (2.4) | 1.03, sb |
| 5.70, d (2.3) | 5.71, d (2.5) | 5.70, d (2.5) | 5.70, s | 5.70, d (5.7) | ||
| 18 | 1.45, s | 1.52, s | 1.54, s | 1.39, s | 1.39, s | 1.61, s |
| 19 | 1.74, s | 1.39, s | 1.32, s | 5.35, s | 5.37, s | 1.54, s |
| 5.29, s | 5.27, s | |||||
| 20 | 1.36, s | 1.25, s | 1.23, s | 1.32, s | 1.33, s | 1.25, s |
| OOH | 7.9, brs | 8.36, brs | ||||
| Position | 1 | 2 | 3 | 4 | 5 | 7 |
|---|---|---|---|---|---|---|
| a Bruker-DRX spectrometer (125 MHz for compounds 1, 3, and 7; 100 MHz for compounds 2, 4 and 5) in CDCl3, chemical shifts (ppm) referred to CDCl3 (δC 77.0).b Exchangeable. | ||||||
| 1 | 33.7, CH | 34.5, CH | 34.6, CH | 34.5, CH | 34.7, CH | 35.6, C |
| 2 | 27.6, CH2 | 26.2, CH2 | 26.2, CH2 | 26.9, CH2 | 25.7, CH2 | 140.9, CH |
| 3 | 84.5, CH | 83.5, CH | 83.7, CH | 82.1, CH | 82.0, CH | 125.0, CH |
| 4 | 73.9, C | 72.6, C | 72.6, C | 72.3, C | 72.6, C | 41.4, CH2 |
| 5 | 38.4, CH2 | 42.4, CH2 | 41.7, CH2 | 30.0, CH2 | 30.6, CH2 | 134.5, C |
| 6 | 22.4, CH2 | 122.5, CH | 120.9, CH | 27.8, CH2 | 26.8, CH2 | 123.4, CH |
| 7 | 128.9, CH | 135.7, CH | 139.2, CH | 72.7, C | 85.1, C | 22.1, CH2 |
| 8 | 138.1, C | 83.9, C | 73.3, C | 146.8, C | 142.0, C | 39.1, CH2 |
| 9 | 75.3, CH | 33.0, CH2 | 38.4, CH2 | 32.2, CH2 | 31.7, CH2 | 134.6, C |
| 10 | 33.3, CH2 | 22.7, CH2 | 23.0, CH2 | 23.4, CH2 | 24.0, CH2 | 125.4, CH |
| 11 | 60.8, CH | 65.5, CH | 65.8, CH | 64.0, CH | 63.6, CH | 24.3, CH2 |
| 12 | 59.1, C | 59.4, C | 59.4, C | 59.3, C | 59.2, C | 37.2, CH2 |
| 13 | 34.5, CH2 | 34.8, CH2 | 34.8, CH2 | 34.3, CH2 | 34.4, CH2 | 59.5, C |
| 14 | 33.4, CH2 | 29.7, CH2 | 29.5, CH2 | 28.8, CH2 | 30.4, CH2 | 58.8, CH |
| 15 | 139.9, C | 139.2, C | 139.2, C | 139.9, C | 139.7, C | 42.6, CH2 |
| 16 | 167.2, C | 166.0, C | 166.0, C | 167.2, C | 167.0, C | 26.6, CH3b |
| 17 | 128.4, CH2 | 127.4, CH2 | 127.3, CH2 | 128.3, CH2 | 128.4, CH2 | 30.0, CH3b |
| 18 | 24.7, CH3 | 25.2, CH3 | 25.2, CH3 | 24.6, CH3 | 24.6, CH3 | 17.7, CH3 |
| 19 | 10.6, CH3 | 25.1, CH3 | 31.2, CH3 | 110.0, CH2 | 112.7, CH2 | 15.6, CH3 |
| 20 | 15.5, CH3 | 15.3, CH3 | 15.2, CH3 | 15.6, CH3 | 15.6, CH3 | 18.2, CH3 |
The molecular formula of compound 4, 15(17)-dehydromanaarenolide A, was deduced to be C20H30O6 by HRESIMS, 13C NMR and DEPT spectra. The diagnostic 1H NMR signal appearing at δH 8.36 (s) suggested the presence of a hydroperoxy group in 4. A comparison of overall 1H and 13C NMR data (Table 1) of 4 with those of the model compound, manaarenolide A, revealed great similarities with the exception that the methyl group (δH 1.37; δC 17.1) in manaarenolide A replaced by a terminal double bond (δH 6.48 and 5.70; δC 128.4) in 4. Therefore, the structure of 4 was identified as 15(17)-dehydromanaarenolide A.
Compound 5, 15(17)-dehydromanaarenolide C, showed NMR spectroscopic data extremely similar to those of 4 (Tables 1 and 2), except for the upfield shifted (δC 72.7 δH 4.32) at C-7 with respect to that of 4 (δC 85.1 δH 4.50). Therefore, the hydroperoxyl group attached at C-7 in 4 was assumed to be converted to a hydroxyl group in 5, which was further supported by a loss of sixteen mass units than that of 4 as a HRESIMS peak at m/z 373.1985 (M + Na)+ observed in 5. In fact, it also differs from another model compound, manaarenolide C, only by the presence of a double bond (δH 6.50 and 5.70; δC 128.3) in 5, instead of a methyl group (δH 1.37; δC 17.3) at C-15/C-17 position. The configurations of 5 were elucidated to be the same as those of manaarenolide C by comparison of their 13C NMR data. Accordingly, the structure of 5 was determined to be a 15(17)-dehydro derivative of manaarenolide C.
The molecular formula of compound 6 was established as C22H32O6 by HRESIMS (m/z 415.2116 [M + Na]+). A literature investigation revealed that compound 6 is identical in all aspects, except for the optical rotation sign and magnitude, to a known cembranoid, flexilarin A, earlier reported from S. flexibilis.4 An opposite optical rotation observed of 6 {[α]20D − 38.0 (c 0.05, CH2Cl2)} as that of flexilarin A {[α]20D + 20.0 (c 0.20, CH2Cl2)}, indicated compound 6 to be an enantiomer of flexilarin A. In fact, we recorded twice the [α]D value of compound 6 to confirm the correctness of our measurement. As a consequence, the structure of compound 6 was tentatively named as epi-flexilarin A.
The HREIMS spectrum of 7 exhibited a molecular peak at m/z 288.2466, consistent with the molecular formula C20H32O, implying five degrees of unsaturation. The 13C NMR and DEPT data of 7 showed the presence of two trisubstituted double bonds, one 1,1-disubstitued double bond and one trisubstituted epoxide, which accounted for four degrees of unsaturation. The remaining degree of unsaturation was assigned to be one ring in the molecule. The 1H NMR spectrum of 7 displayed methyl singlets at δH 1.02 (s, 3H), 1.10 (s, 3H), 1.54 (s, 3H), 1.61 (s, 3H), 1.25 (s, 3H), which were assigned to two tertiary, two vinyl and a hydroxyl-bearing methyl groups. Analysis of the 1H-1H COSY spectra readily identified four spin–spin systems: H-2 (δH 5.40) to H-3 (δH 5.46), H-3 to H-4 (δH 2.62); H-6 (δH 5.03) to H-7 (δH 2.07 and 1.95), H-7 to H-8 (δH 2.13 and 1.92); H-10 (δH 5.01) to H-11 (δH 2.14 and 1.92), H-11 to H-12 (δH 1.51) and H-14 (δH 2.79) to H-15 (δH 1.71 and 1.35). In the HMBC spectrum of 7, the cross-peaks from Me-16 (δH 1.10) and Me-17 (δH 1.13) to C-1 (δC 35.6), C-2 (δC 140.9) and C-15 (δC 42.6) determined the position of these two tertiary methyl group; the correlations from Me-18 (δH 1.61) to C-4 (δC 41.4), C-5 (δC 134.5) and C-6 (δC 123.4), and from Me-19 (δH 1.54) to C-8 (δC 39.1), C-9 (δC 134.6) and C-10 (δC 125.4) indicated the position of the two vinyl methyl groups; finally, the position of the epoxide ring located at C-13/14 was supported by the cross-peaks from Me-20 (δH 1.25) to C-13 (δC 59.5), C-14 (δC 58.8) and C-15. On the basis of the above evidences, the molecular framework of 7 could be established as an extremely unusual 15-membered macrocyclic diterpenoid. Checking the literature revealed that there was only one natural product, flexibilene, which was previously isolated from the same soft coral species,6,21,22 possessing the same skeleton. In fact, only difference between 7 and flexibilene was that the trisubstituted double bond at Δ13/14 in flexibilene was replaced by an epoxy group (δH 2.79; δC 59.5 and 58.8) in 7. Furthermore, on the basis of the 13C NMR chemical shift for CH3-20 (δC 18.2, <20 ppm),23 the configuration of the epoxide ring was defined as trans. In addition, the E geometries for three olefins at Δ2/3,5/6,9/10, the same as that of flexibilene, were further secured by either the large coupling constant observed between H-2 and H-3 (J = 15.8 Hz) or 13C NMR chemical shift for CH3-18 (δC 17.7, <20 ppm) and CH3-19 (δC 15.6, <20 ppm).24 Thus, structure of 7, named epoxyflexibilene, was unambiguously determined.
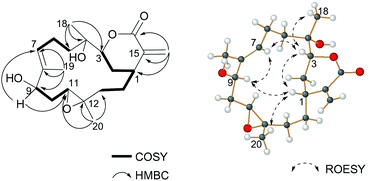
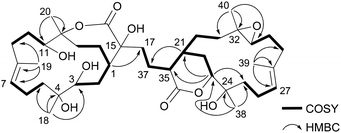
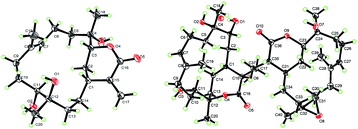
Sinulaflexiolide L (8) was obtained as colorless crystals, mp 240–242 °C. Analysis of HRESIMS, 13C NMR and DEPT data revealed a molecular formula of C40H64O10. Its 1H-NMR spectrum (Table 3) showed signals attributable to two lactonic carbonyl groups (δC 183.4, 173.8), a lactonic methine [δC 84.2 (CH) and δH 4.26 (1H, d, J = 9.9 Hz)], a lactonic quaternary carbon (δC 88.2), two trisubstituted double bonds [δC 128.5 (CH), 133.5 (C); 126.5 (CH), 133.4 (C) and δH 5.42 (1H, d, J = 9.5 Hz); 5.28 (1H, t, J = 7.1 Hz)], three tertiary hydroxyls [δC 75.1 (C), 73.9 (C) and 80.6 (C)], two secondary hydroxyls [δC 75.4 (CH), 66.9 (CH) and δH 4.35 (1H, d, J = 7.9 Hz), 4.68 (1H, t, J = 8.8 Hz)], a epoxymethine [δC 63.0 (CH) and δH 3.02 (1H, t, J = 5.7 Hz)], a epoxydic quaternary carbon (δC 59.0), three methines and sixteen methylenes. The presence of six tertiary methyl groups in the molecule was deduced by 1H NMR signals at δH 1.88 (3H, s H3-19), 1.69 (3H, s H3-18), 1.65 (3H, s H3-20), 1.61 (3H, s H3-38), 1.54 (3H, s H3-39) and 1.43 (3H, s H3-40), respectively. Based on the above observations, 8 was suggested to be a biscembranoid similar to those previously reported from soft coral of the same genus, such as sinuflexlin3 and sinulaflexiolide A (13).8 All NMR resonances of 8 were assigned by analysis of 2D NMR spectra as reported in Table 3. Selected 1H-1H COSY and HMBC correlations of compound 8 are reported in Fig. 3. Fortunately, a single-crystal of 8 suitable for X-ray diffraction analysis was obtained from the methanol solution. The X-ray result (Fig. 4) clearly indicated that compound 8 could biogenetically derive from two different cembranoid units, 9 and 12, probably through an oxo-Diels–Alder reaction and a subsequent hydrolysis as suggested for another biscembranoid, sinuflexlin3 deriving from two units of 9.
| Position | δC, type | δH, mult. (J in Hz) |
|---|---|---|
| a Bruker-DRX-500 spectrometer (500 MHz for 1H NMR) in Pry-d5, chemical shifts (ppm) referred to pryidine (δH 8.72, 7.57, 7.20) residual signal. Proton coupling constants (J) in Hz are given in parentheses.b Bruker-DRX-400 spectrometer (125 MHz for 13C NMR) in Pry-d5, chemical shifts (ppm) referred to pryidine (δC 149.9, 135.5, 123.5).c Exchangeable. | ||
| 1 | 38.6, CH | 2.63, m |
| 2 | 27.9, CH2 | 2.34, m; 1.64, m |
| 3 | 75.4, CH | 4.35, d (7.9) |
| 4 | 75.1, C | |
| 5 | 39.8, CH2 | 2.14, m; 1.80, m |
| 6 | 23.8, CH2 | 2.50, m; 2.30, m |
| 7 | 128.5, CH | 5.42, d (9.5) |
| 8 | 133.4, C | |
| 9 | 36.5, CH2 | 2.26, m |
| 10 | 25.3, CH2 | 1.69, m |
| 11 | 66.9, CH | 4.68, d (8.8) |
| 12 | 88.2, C | |
| 13 | 32.4, CH2 | 2.14, m; 1.84, m |
| 14 | 28.6, CH2 | 2.51, m; 1.92, m |
| 15 | 80.6, C | |
| 16 | 183.4, C | |
| 17 | 36.2, CH2 | 2.54, m; 2.46, m |
| 18 | 25.2, CH3 | 1.69, s |
| 19 | 16.2, CH3 | 1.88, s |
| 20 | 21.9, CH3 | 1.65, s |
| 3-OH | 5.24, brs | |
| 4-OH | 6.38, brsc | |
| 11-OH | 6.49, brs | |
| 21 | 32.6, CH | 2.37, m |
| 22 | 28.0, CH2 | 2.24, m; 1.73, m |
| 23 | 84.2, CH | 4.26, d (9.9) |
| 24 | 73.9, C | |
| 25 | 40.2, CH2 | 1.93, m |
| 26 | 23.2, CH2 | 2.22, m; 1.95, m |
| 27 | 126.5, CH | 5.28 t (7.1) |
| 28 | 133.5, C | |
| 29 | 35.9, CH2 | 2.07, m |
| 30 | 25.6, CH2 | 1.86, m; 1.62, m |
| 31 | 63.0, CH | 3.02, t (5.7) |
| 32 | 59.0, C | |
| 33 | 35.2, CH2 | 1.97, m; 1.32, m |
| 34 | 31.8, CH2 | 1.85, m; 1.05, m |
| 35 | 48.1, CH | 2.42, m |
| 36 | 173.8, C | |
| 37 | 36.0, CH2 | 2.33, m |
| 38 | 25.0, CH3 | 1.61, s |
| 39 | 16.1, CH3 | 1.54, s |
| 40 | 16.0, CH3 | 1.43, s |
| 15-OH | 7.32, brs | |
| 24-OH | 5.11, brsc | |
With the biogenetic consideration, and by analogy to 9, the absolute configurations of 8 at C-21, C-23, C-24, C-31 and C-32 were tentatively assigned as R, R, S, S, and S, respectively, and consequently, the absolute configuration of 8 was suggested as (1R, 3R, 4S, 11S, 12R, 15S, 21R, 23R, 24S, 31S, 32S, 35S).
The abundant production and accumulation of cembrane terpenes (9, 10, 12 and 13, 4 analogues reported in this paper) in this specimen of S. flexibilis is intriguing. The highest production of flexibilide (9) in the title animalis not only widely distributed in most colonies of S. flexibilis, but also well-known for its antifouling, allelopathy and cytotoxic activity.9 Thus, all the compounds related to 9 (1–9 and 13) were tested against a panel of tumor cell lines including HL-60, A-549 and HCT-116, as well as X-box binding protein 1 (XBP1), a novel targeting as anti-tumor strategy.25 The result revealed that only flexibilide (9) showed significant activity. In particular, 9 exhibited potent anti-tumor activity targeting the inositol-requiring 1/X-box-binding protein 1 (IRE1/XBP1) signaling pathway, with an IC50 value of 4.10 μg mL−1.
Experimental
General experimental procedures
Optical rotations were measured on a Perkin-Elmer 241MC polarimeter. IR spectra were recorded on a Nicolet-Magna FT-IR 750 spectrometer. HRESIMS spectra were recorded on a Q-TOF Micro LC-MS-MS mass spectrometer. The NMR spectra were measured on a Bruker DRX-400/500 spectrometer with the residual CHCl3 (δH 7.26 ppm, δC 77.0 ppm) or pyridine (δH 8.72, 7.57, 7.20 ppm, δC 149.9, 135.5, 123.5 ppm) as internal standard. Chemical shifts are expressed in δ (ppm) and coupling constants (J) in Hz. 1H and 13C NMR assignments were supported by 1H-1H COSY, HSQC, HMBC and ROESY experiments. Commercial Silica gel (Qing Dao Hai Yang Chemical Group Co., 200–300 and 400–600 mesh) and Sephadex LH-20 (Amersham Biosciences) were used for column chromatography. Reversed phase HPLC (Agilent 1100 series liquid chromatography using a VWDG1314A detector at 210 nm and a semi-preparative ODS-HG-5 [5 μm, 10 mm (i.d.) × 25 cm] column) was also employed.Biological material
The soft corals S. flexibilis was collected by scuba at Yalong Bay, Hainan Province, China, in February 26, 2006, at a depth of −15 to −20 m, and identified by Professor R.-L. Zhou of South China Sea Institute of Oceanology, Chinese Academy of Sciences. The voucher sample is deposited at the Shanghai Institute of Materia Medica, CAS, under registration no. YAL-19.Extraction and separation
The lyophilized bodies of S. flexibilis (220 g, dry weight) were minced into pieces and exhaustively extracted with Me2CO at room temperature (3 × 1 L). The solvent-free Me2CO extract was partitioned between Et2O and H2O. The organic phase was evaporated under reduced pressure to give a dark brown residue (10 g), which was subjected to Si gel column chromatography (CC) and eluted with petroleum ether (PE) in Et2O (0–100%, gradient) to yield 9 fractions (A–I). Fraction B was chromatographed over Sephadex LH-20 eluting with PE/CHCl3/MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by silica gel CC (PE/Me2CO, 200
1), followed by silica gel CC (PE/Me2CO, 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 50
1 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 7 (7.3 mg). Fraction E eluted with PE/Et2O (6
1) to afford 7 (7.3 mg). Fraction E eluted with PE/Et2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to yield 9 (2001.7 mg). Fraction F was divided into six subfractions (F1–F6) by silica gel CC (PE/Me2O, 7
4) to yield 9 (2001.7 mg). Fraction F was divided into six subfractions (F1–F6) by silica gel CC (PE/Me2O, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 5
3 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), each of which was chromatographed over Sephadex LH-20 eluting with PE/CHCl3/MeOH (2
5), each of which was chromatographed over Sephadex LH-20 eluting with PE/CHCl3/MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by RP-HPLC (MeOH/H2O, 58
1), followed by RP-HPLC (MeOH/H2O, 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42; MeOH/H2O, 65
42; MeOH/H2O, 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35; MeOH/H2O, 68
35; MeOH/H2O, 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32, respectively) to afford 2 (3.1 mg), 4 (2.3 mg), 6 (1.5 mg), 11 (3.9 mg). Fraction G eluted with silica gel CC (CHCl2/MeOH, 400
32, respectively) to afford 2 (3.1 mg), 4 (2.3 mg), 6 (1.5 mg), 11 (3.9 mg). Fraction G eluted with silica gel CC (CHCl2/MeOH, 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 90
1 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give seven subfractions (G1–G7), each of which was separated by a column of Sephadex LH-20 eluting with PE/CHCl3/MeOH (2
1) to give seven subfractions (G1–G7), each of which was separated by a column of Sephadex LH-20 eluting with PE/CHCl3/MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and RP-HPLC (MeOH/H2O, 49
1) and RP-HPLC (MeOH/H2O, 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51; MeOH/H2O, 75
51; MeOH/H2O, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, respectively) to yield 1 (1.4 mg), 5 (2.0 mg), 10 (2.8 mg), 12 (9.0 mg), 13 (2.4 mg). Fraction H was chromatographed over Sephadex LH-20 eluting with PE/CHCl3/MeOH (2
25, respectively) to yield 1 (1.4 mg), 5 (2.0 mg), 10 (2.8 mg), 12 (9.0 mg), 13 (2.4 mg). Fraction H was chromatographed over Sephadex LH-20 eluting with PE/CHCl3/MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by RP-HPLC (MeOH/H2O, 54
1), followed by RP-HPLC (MeOH/H2O, 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46) to afford 3 (1.9 mg). Finally, fraction I eluted with silica gel CC (CHCl2/MeOH, 9
46) to afford 3 (1.9 mg). Finally, fraction I eluted with silica gel CC (CHCl2/MeOH, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and was further purified by RP HPLC (MeOH/H2O, 50
1) and was further purified by RP HPLC (MeOH/H2O, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to afford 8 (2.0 mg).
50) to afford 8 (2.0 mg).
Acetylation of sinuflexolide (10)
To a dry pyridine (0.5 mL) of 10 (1.0 mg) was added 1 drop of Ac2O. The mixture was stirred at room temperature overnight, and then stopped by adding 1 drop of H2O. The crude acetylated products, after evaporating the solvent in vacuo, were purified on silica gel column chromatography (PE/Me2CO in gradient), to afford the expected di-acetate 11. Physical and spectroscopic data of this product were found to be in full agreement with those of the natural product 11.Reduction of 15,17-didehydromanaarenolide E (2)
2 (1.0 mg) was stirred with 4 mg of triphenylphosphine in 2.5 mL of ether for 4 h at room temperature. After evaporation of excess reagent, the residue was separated by short column chromatography on silica gel (CH2Cl2–MeOH in gradient) to give a reduced product of 2. Physical and spectroscopic data of this product were found to be in full agreement with those of the natural product 3.X-ray crystallographic studies of sinuflexolide (10)
Colorless block crystals of 10 were obtained by recrystallization in Me2CO–H2O mixtures. Crystal data were obtained on a Bruker APEX-II CCD diffractometer with monochromated Cu Kα radiation (λ = 1.54178 Å). The structure was solved by direct methods (SHELXS-97) and refined using full-matrix least squares difference Fourier techniques. All non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were placed in idealized positions and refined as riding atoms with the relative isotropic parameters.X-ray crystallographic studies of sinulaflexiolide L (8)
Colorless block crystals of 8 were obtained by recrystallization in MeOH–H2O mixtures. Crystal data were obtained on a Bruker APEX-II CCD diffractometer with monochromated Mo Kα radiation. The structure was solved by direct methods (SHELXS-97) and refined using full-matrix least squares difference Fourier techniques. All non-hydrogen atoms were refined anisotropically, and all hydrogen atoms were placed in idealized positions and refined as riding atoms with the relative isotropic parameters.Cytotoxicity bioassays
The cytotoxicities of compounds 1–9 and 13 against human promyelocytic leukemia cell lines HL60, human lung adenocarcinoma A-549, human colon carcinoma HCT-116 cell lines, and IRE1/XBP1 signaling pathway were evaluated by using the MTT,26 SRB27 and B16-F10-XBP1-DBD-Luc28 methods, respectively, according to the protocols described in previous literature.Conclusion
In conclusion, eight new and five known terpenoids belonging to three different structural classes were isolated from the Hainan soft coral S. flexibilis. The first structural class, including six new (1–6) and three known cembranoids (9–11), all possesses a typical common α-methylene-δ-lactone group. Since these cembranoids are mainly isolated from soft corals of genus Sinularia,2–6,8,18,20,29 they are commonly regarded as chemotaxonomic markers of this genus. Further, the absolute configurations of two known compounds, sinuflexolide (10)2 and its acetylated derivative, 11-acetylsinuflexolide (11),18 both previously isolated from the same species of Taiwan origin, have been unambiguously determined firstly by Cu-Kα X-ray diffraction analysis [Flack parameters: −0.1(2)] on the crystal of 10 and then successively by chemical conversion of 10 to 11 in the present work, as a completion for their full structural elucidation. Epoxyflexibilene (7), represents the second example of 15-membered ring macrocyclic diterpenoid that have ever discovered from marine organism. In fact, the first 15-membered diterpene, flexibilene, a formal precursor of 7, was found from the same species but three different locations, Kisser Island,21 the Great Barrier Reef,6 and Indian Ocean.22 Interestingly, this is the first time that such kind of macrocyclic diterpenoid was isolated from the South China Sea collection. The third structural class, exemplified by sinulaflexiolide L (8), displays the extremely rare dimeric cembrane skeleton formally connected through C–C single bond of two cembrane monomers. It may be worth to point out that up to now, there are only two biscembranoids, sinuflexlin from Taiwan S. flexibilis3 and sinulaflexiolide A (13) from Hainan S. flexibilis,8 possessing the same skeleton, were reported. Compound 8 is the third member of this unusual biscembranoids family.The discovery of new compounds 1–8 from S. flexibilis collected in the South China Sea is not only an addition of a diverse and complex series of terpenoids to this species, but also provides the evidence of the powerful adaptive capacity of this animal towards various marine ecological environment by the production of diverse secondary metabolites. Further studies would be intriguing and challenging on the understanding of this chemical ecological influence in a genetic point of view, and exploring the biological role of these second metabolites in the life cycle of the title animal. Chemical methods, such as total synthesis or structure modifications, could also be interesting to be involved in the structure determination and structure–activity relationship study of these structurally intriguing and biologically active terpenoids.
Acknowledgements
This research work was financially supported by the National Marine ‘863’ Project (no. 2013AA092902), the Natural Science Foundation of China (no. 81273430), the SKLDR/SIMM Project (no. 1501ZZ-01), NSFC-Shandong Joint Fund for Marine Science Research Centers (Grant no. U1406402) and was partially funded by the EU 7th Framework Programme-IRSES Project (no. 246987).Notes and references
- W.-T. Chen, Y. Li and Y.-W. Guo, Acta Pharm. Sin. B, 2012, 2, 227–237 CrossRef CAS PubMed.
- C.-Y. Duh, S.-K. Wang, H.-K. Tseng, J.-H. Sheu and M.-Y. Chiang, J. Nat. Prod., 1998, 61, 844–847 CrossRef CAS PubMed.
- C.-Y. Duh, S.-K. Wang, H.-K. Tseng and J.-H. Sheu, Tetrahedron Lett., 1998, 39, 7121–7122 CrossRef CAS.
- Y.-S. Lin, C.-H. Chen, C.-C. Liaw, Y.-C. Chen, Y.-H. Kuo and Y.-C. Shen, Tetrahedron, 2009, 65, 9157–9164 CrossRef CAS PubMed.
- B.-W. Chen, C.-H. Chao, J.-H. Su, C.-Y. Huang, C.-F. Dai, Z.-H. Wen and J.-H. Sheu, Tetrahedron Lett., 2010, 51, 5764–5766 CrossRef CAS PubMed.
- R. Kazlauskas, P. T. Murphy, R. J. Wells, P. Schonholzer and J. C. Coll, Aust. J. Chem., 1978, 31, 1817–1824 CrossRef CAS.
- P. P. Guerrero, R. W. Read, M. Batley and G. C. Janairo, J. Nat. Prod., 1995, 58, 1185–1191 CrossRef CAS.
- T. Wen, Y. Ding, Z.-W. Deng, L.-P. Van Ofwegen, P. Proksch and W.-H. Lin, J. Nat. Prod., 2008, 71, 1133–1140 CrossRef CAS PubMed.
- J. C. Coll, Chem. Rev., 1992, 92, 613–631 CrossRef CAS.
- T.-R. Su, J.-J. Lin, C.-C. Chiu, J. Y. F. Chen, J.-H. Su, Z.-J. Cheng, W.-I. Hwang, H.-H. Huang and Y.-J. Wu, Electrophoresis, 2012, 33, 1139–1152 CrossRef CAS PubMed.
- T. L. Aceret, J. C. Coll, Y. Uchio and P. W. Sammarco, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 1998, 120, 121–126 CrossRef CAS.
- P. J. Buckle, B. A. Baldo and K. M. Taylor, Agents Actions, 1980, 10, 361–367 CrossRef CAS.
- L.-F. Liang, X.-J. Wang, H.-Y. Zhang, H.-L. Liu, J. Li, L.-F. Lan, W. Zhang and Y.-W. Guo, Bioorg. Med. Chem. Lett., 2013, 23, 1334–1337 CrossRef CAS PubMed.
- S. Qin, H. Huang and Y.-W. Guo, J. Asian Nat. Prod. Res., 2008, 10, 1075–1079 CrossRef CAS PubMed.
- Y. Li, H. Huang and Y.-W. Guo, Nat. Prod. Res., 2008, 22, 1359–1364 CrossRef CAS PubMed.
- Y. Li, A.-H. Gao, H. Huang, J. Li, E. Mollo, M. Gavagnin, G. Cimino, Y.-C. Gu and Y.-W. Guo, Helv. Chim. Acta, 2009, 92, 1341–1348 CrossRef CAS.
- Y. Li, M. Carbone, R. M. Vitale, P. Amodeo, F. Castelluccio, G. Sicilia, E. Mollo, M. Nappo, G. Cimino, Y.-W. Guo and M. Gavagnin, J. Nat. Prod., 2010, 73, 133–138 CrossRef CAS PubMed.
- C.-C. Su, B.-S. Wong, C. Chin, Y.-J. Wu and J.-H. Su, Int. J. Mol. Sci., 2013, 14, 4317–4325 CrossRef CAS PubMed.
- J.-Y. Su, R.-L. Yang, Y.-Y. Kuang and L.-M. Zeng, J. Nat. Prod., 2000, 63, 1543–1545 CrossRef CAS PubMed.
- J.-H. Su, A. F. Ahmed, P.-J. Sung, C.-H. Chao, Y.-H. Kuo and J.-H. Sheu, J. Nat. Prod., 2006, 69, 1134–1139 CrossRef CAS PubMed.
- A. S. R. Anjaneyulu, K. S. Sagar and G. V. Rao, J. Nat. Prod., 1997, 60, 9–12 CrossRef CAS.
- M. Herin, M. Colin and B. Tursch, Bull. Soc. Chim. Belg., 1976, 85, 801–803 CrossRef CAS.
- B. N. Ravi and D. John Faulkner, J. Org. Chem., 1978, 43, 2127–2131 CrossRef CAS.
- T. Iwagawa, R. Nakashima, K. Takayama, H. Okamura, M. Nakatani, M. Doe and K. Shibata, J. Nat. Prod., 1999, 62, 1046–1049 CrossRef CAS PubMed.
- A. C. Koong, V. Chauhan and L. Romero-Ramirez, Cancer Biol. Ther., 2006, 5, 756–759 CrossRef CAS.
- M. C. Alley, D. A. Scudiero, A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker and M. R. Boyd, Cancer Res., 1988, 48, 589–601 CAS.
- P. A. Skehan, R. Storeng, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82, 1107–1112 CrossRef CAS PubMed.
- L.-X. Fan, A.-H. Gao, Y.-B. Zhou and J. Li, China Biotechnol., 2012, 32, 73–80 CAS.
- A. J. Weinheimer, J. A. Matson, M. B. Hossain and D. Vanderhelm, Tetrahedron Lett., 1977, 34, 2923–2926 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Spectra for compounds 1–8, including 1H and 13C NMR, COSY, HMBC, HSQC, HRESIMS. CCDC 1040659 and 1040484. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra01151e |
| This journal is © The Royal Society of Chemistry 2015 |