Lewis acid catalyzed C-3 alkylidenecyclopentenylation of indoles: an easy access to functionalized indoles and bisindoles†
S. Sarath Chandab,
B. S. Sasidharab,
Praveen Prakashb,
P. Sasikumarb,
P. Preethanujb,
Florian Jaroschikc,
Dominique Harakatc,
Jean-Luc Vassec and
K. V. Radhakrishnan*ab
aAcademy of Scientific and Innovative Research (AcSIR), New Delhi 110001, India. E-mail: radhu2005@gmail.com
bOrganic Chemistry Section, National Institute for Interdisciplinary Science and Technology (CSIR), Trivandrum 695019, India
cUniversité de Reims, 51687 Reims Cedex 2, France
First published on 16th March 2015
Abstract
A Lewis acid catalyzed C-3 alkylidenecylopentenylation of indoles through the ring opening of pentafulvene derived diazabicyclic olefins has been developed. The present protocol offers an efficient route toward the synthesis of indole and bisindole derivatives. The role of the hydrazine group, as a reaction carrier in the strategy has also been demonstrated by the stepwise synthesis of functionalized bisindole.
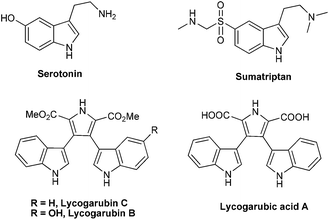
Indole, an important nitrogen containing heterocyclic scaffold, is one of the primary building blocks of many natural products, biologically active molecules and functional materials.1 Moreover, indole is the key component in many pharmaceutical agents such as triptan and its derivatives, a class of psychoactive drugs used in the treatment of 5HT receptor related disorders (Fig. 1).2 Owing to the great prevalence of the indole nucleus, enormous research effort has been devoted towards the synthesis and functionalization, especially at the C-3 position, of this privileged core.3 Various synthetic methodologies, involving Lewis and Brønsted acids, organocatalysts or transition metal catalysts, have been developed by different research groups for the selective C-3 functionalization of indoles.4 In 2001, Kobayashi et al. achieved the C-3 cyclopentenylation of indoles through a Lewis acid/surfactant catalyzed Friedel–Crafts type conjugate addition in aqueous medium.5 Later, King and coworkers explored the Lewis acid catalyzed Michael addition of indoles to cyclopent-2-enone for the preparation of 3-cis-(3-aminocyclopentenyl)indoles as potent inhibitors of hSERT.6 Among indole derivatives, bisindole moieties are present in several natural alkaloids7 and many of these compounds show promising medicinal properties (Fig. 1).8 So the design and development of new atom economic methods for construction of bisindole derivatives remains as a highly demanding strategy in organic synthesis. Herein, we disclose a Lewis acid catalyzed ring-opening of pentafulvene derived bicyclic olefins with N-alkyl as well as free (NH) indoles toward the efficient synthesis of mono and bisindolyl functionalized alkylidenecyclopentenes.
In various cycloaddition reactions, pentafulvenes, a cyclic cross conjugated system, have been well explored as a 2π, 4π or 6π component for the construction of numerous biologically relevant molecules.9 Additionally, desymmetrization of diazabicyclic olefins under transition metal catalysis or acid catalysis has been developed as an efficient protocol by several research groups,10 including our laboratory for the synthesis of highly functionalized cyclopentene derivatives.11
As part of our continuous interest in the chemistry of strained norbornene derivatives, we have utilized diazabicyclic olefins derived from different pentafulvenes as a simple precursor for the synthesis of substituted alkylidenecyclopentenes and complex heterocyclic scaffolds in the presence of a palladium catalyst or Lewis acid. In our previous report we have demonstrated a Lewis acid catalyzed ring-opening of pentafulvene derived diazabicyclic olefins using various ortho-functionalized aryl iodides such as 2-iodoanilines, 2-iodophenols and 2-iodobenzene thiols and aliphatic alcohols to access a variety of trans-3,4-disubstituted alkylidenecyclopentenes.12 In the same report, a palladium/Lewis acid mediated transformation of pentafulvene derived diazabicyclic olefins has also been described for the synthesis of novel spiropentacyclic motifs with indoline/dihydrobenzothiophene and pyrazolidine fused to the cyclopentene core. As a perpetuation of our ongoing investigations in the area of strained bicyclic olefins, we have decided to undertake the Lewis acid catalyzed desymmetrization of pentafulvene derived diazabicyclic olefins by employing biologically significant indoles as nucleophiles. The developed method successfully leads to the C-3 functionalization of indoles with alkylidenecyclopentenes, along with the formation of bisindole derivatives.
We initiated our investigation by the treatment of pentafulvene derived diazabicyclic olefin 1a (1.2 equiv.) with N-methylindole 2b (1 equiv.) in the presence of Sc(OTf)3 (2 mol%) in toluene at room temperature (Scheme 1). After 4 h, the reaction afforded the desired trans-3,4-disubstituted alkylidene cyclopentene derivative 3ab in 44% yield. The structure of 3ab was established by the usual spectroscopic techniques and also based on comparison with our previous report.12 Furthermore, the structure and stereochemistry of the product was confirmed by single crystal X-ray analysis of a similar derivative 3eb (see ESI†).
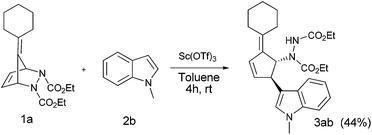 | ||
| Scheme 1 Lewis acid catalyzed C-3 functionalization of N-methylindole 2b with diazabicyclic olefin 1a. | ||
Further screening of solvents such as DMF, THF, 1,2-dichloroethane, DCM and CH3CN revealed that CH3CN was the most favorable medium for the transformation. Astonishingly, when CH3CN was employed as the solvent, the bisindolyl functionalized alkylidene cyclopentene 4ab was observed along with the expected 3,4-disubstituted alkylidene cyclopentene 3ab (Scheme 2). Various Lewis acids were also tested for the ring-opening of 1a with 2b in acetonitrile. Among them, Sn(OTf)2 and Fe(OTf)3 provided the product 3ab in comparable yields. During optimization studies, we perceived that the change in equivalents of starting materials 1a or 2b played a crucial role in the outcome of the reaction. Use of 2 equiv. of N-methylindole 2b resulted in the formation of bisindole product 4ab (58% yield) in excess over 3ab (31% yield) (entry 16). Under optimal conditions (2 mol% Sc(OTf)3 in CH3CN), the reaction could be finely tuned towards the formation of alkylidenecyclopentenyl derivative of indole 3ab or bisindole 4ab by simply altering the equivalents of starting materials 1a or 2b.
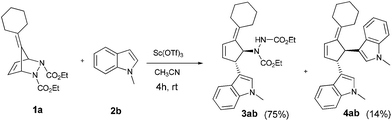 | ||
| Scheme 2 Sc(OTf)3 catalyzed C-3 functionalization of N-methylindole 2b with diazabicyclic olefin 1a in acetonitrile. | ||
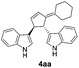
Under the optimized catalytic conditions for the preparation of alkylidenecyclopentenyl derivative of indole (Table 1, entry 6) and bisindole (Table 1, entry 16), we examined the scope of different olefins and indoles (Table 2). Diazabicyclic alkenes 1a–d easily underwent ring opening with 1H-indole 2a and gave the corresponding indole derivatives 3aa–da and bisindole 4aa in good to moderate yields (entries 1–4). To demonstrate the generality of the reaction, several C-1, C-2 and C-5 substituted indoles 2b–f were subjected to C-3 alkylidenecyclopentenylation. Reaction was found to be compatible to a variety of indoles having substituents such as –F, –OH, –NO2 etc. and yielded the C-3 functionalized indoles and bisindoles (entries 5–9).
| Entry | Lewis acid | Solvent | Yield (%) | |
|---|---|---|---|---|
| 3ab | 4ab | |||
| a Reaction conditions: alkene (1.2 equiv.), N-methylindole (1 equiv.), catalyst (2 mol%), solvent (2 mL), at rt. for 4 h.b Reaction in the presence of 1.0 equiv. of alkene and 2 equiv. of N-methylindole. | ||||
| 1 | Sc(OTf)3 | Toluene | 44 | — |
| 2 | Sc(OTf)3 | DMF | 38 | — |
| 3 | Sc(OTf)3 | THF | 30 | — |
| 4 | Sc(OTf)3 | DCE | 65 | 5 |
| 5 | Sc(OTf)3 | DCM | 58 | Trace |
| 6 | Sc(OTf)3 | CH3CN | 75 | 14 |
| 7 | Yb(OTf)3 | CH3CN | 37 | — |
| 8 | Zn(OTf)2 | CH3CN | Trace | — |
| 9 | La(OTf)3 | CH3CN | 35 | — |
| 10 | Cu(OTf)2 | CH3CN | 46 | — |
| 11 | Sn(OTf)2 | CH3CN | 70 | 8 |
| 12 | Fe(OTf)3 | CH3CN | 62 | 6 |
| 13 | AgOTf | CH3CN | Trace | — |
| 14 | AlCl3 | CH3CN | 53 | Trace |
| 15 | BF3OEt2 | CH3CN | 36 | — |
| 16b | Sc(OTf)3 | CH3CN | 31 | 58 |
| Entry | Bicyclic olefin | Indole | Product 3 | Product 4 | Yield (%) | |
|---|---|---|---|---|---|---|
| 3 | 4 | |||||
| a Reaction conditons: alkene (1.2 equiv.), indole (1 equiv.), catalyst (2 mol%), solvent (2 mL), at rt. for 4 h.b Reaction in presence of 1 equiv. of alkene and 2 equiv. of indole. | ||||||
| 1 | 1a | 2a | 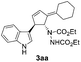 |
 |
73 | 16 |
| 27b | 64b | |||||
| 2 | 1b | 2a | 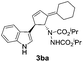 |
4aa | 72 | 16 |
| 14b | 72b | |||||
| 3 | 1c | 2a | 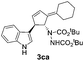 |
4aa | 42 | 14 |
| 27b | 28b | |||||
| 4 | 1d | 2a | 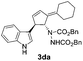 |
4aa | 39 | 12 |
| 17b | 33b | |||||
| 5 | 1a | 2b | 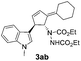 |
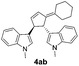 |
75 | 14 |
| 31b | 58b | |||||
| 6 | 1a | 2c | 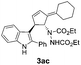 |
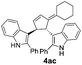 |
78 | 11 |
| 32b | 54b | |||||
| 7 | 1a | 2d | 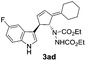 |
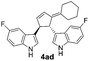 |
61 | 14 |
| 24b | 52b | |||||
| 8 | 1a | 2e | 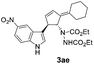 |
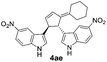 |
59 | 12 |
| 26b | 48b | |||||
| 9 | 1a | 2f | 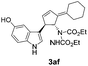 |
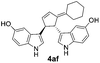 |
56 | 8 |
| 28b | 39b | |||||
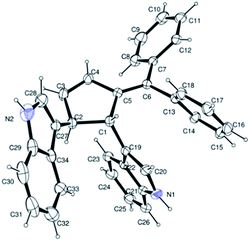
Next, we turned our attention to explore the scope of C-3 functionalization of indoles with diazabicyclic olefins derived from different pentafulvenes (Table 3). Alkylidenecyclopentenylation of indoles proceeds efficiently through the ring opening of diazabicyclic alkenes 1e–h to provide the desired indole and bisindole derivatives. In the case of diphenylfulvene derived bicyclic olefin 1h with indoles 2a and 2b, corresponding bisindole derivatives were formed in 84% and 81% yield respectively (entries 7 and 8). Furthermore, the stereochemistry of the bisindole product 4 was unambiguously confirmed by the single crystal X-ray analysis of compound 4ha (Fig. 2, ESI†).
| Entry | Bicyclic olefin | Indole | Product 3 | Product 4 | Yield (%) | |
|---|---|---|---|---|---|---|
| 3 | 4 | |||||
| a Reaction conditons: alkene (1.2 equiv.), indole (1 equiv.), catalyst (2 mol%), solvent (2 mL), at rt. for 4 h.b Reaction in presence of 1 equiv. of alkene and 2 equiv. of indole. | ||||||
| 1 | 1e | 2a | 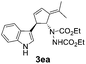 |
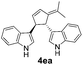 |
69 | 18 |
| 24b | 64b | |||||
| 2 | 1e | 2b | 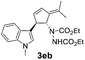 |
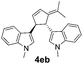 |
65 | 15 |
| 27b | 56b | |||||
| 3 | 1f | 2a | 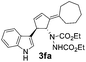 |
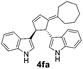 |
70 | 9 |
| 25b | 53b | |||||
| 4 | 1g | 2a | 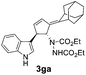 |
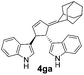 |
66 | 24 |
| 30b | 58b | |||||
| 5 | 1g | 2b | 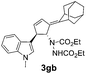 |
 |
70 | 16 |
| 32b | 55b | |||||
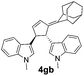
| 6 | 1g | 2c | 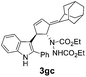 |
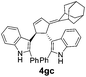 |
66 | 14 |
| 13b | 74b | |||||
| 7 | 1h | 2a | 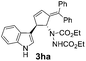 |
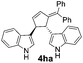 |
62 | 28 |
| 8b | 84b | |||||
| 8 | 1h | 2b | 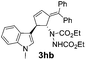 |
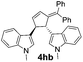 |
68 | 24 |
| 10b | 81b | |||||
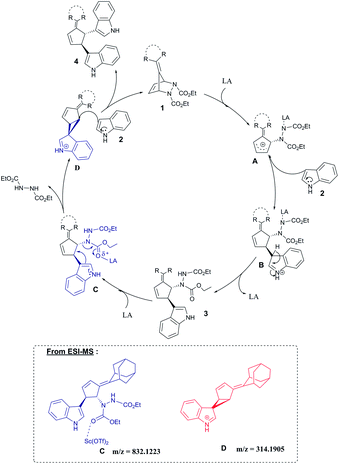
Based on these results we propose a plausible mechanism as shown in Scheme 3. As similar to our previous reports,12 the catalytic cycle is initiated by coordination of the Lewis acid with the carbonyl oxygen of one of the carbamate groups of diazabicyclic olefin 1 and subsequent cleavage of the C–N bond leads to the generation of a transient allylic cation species A. Regioselective nucleophilic attack of indole from the opposite side with respect to the hydrazine moiety of intermediate A delivers trans-3,4-disubstituted alkylidene cyclopentene 3. In the next step, the Lewis acid coordinates with the carbonyl group of the hydrazine moiety, followed by the elimination of the hydrazine group through C–N bond cleavage, resulting in the formation of intermediate D. Attack of the second molecule of indole to intermediate D furnishes the bisindole product 4. Furthermore, ESI-MS studies provided strong supporting evidence for the formation of intermediates C and D (see ESI†).
To confirm the Lewis acid catalyzed generation of an intermediate from 3 by the elimination of hydrazine moiety, we have carried out a reaction with 1 equiv. of 3,4-disubstituted alkylidene cyclopentene 3ha and 1.2 equiv. of indole 2a (Scheme 4). As expected, bisindole product 4ha was obtained in 62% yield, supporting the role of 3,4-disubstituted alkylidene cyclopentene as an intermediate in the course of reaction. It is to be noted that the hydrazine group acts as a key functional moiety in the present atom economic strategy toward the synthesis of functionalized bisindoles. In addition, oxidation of the generated hydrazine could provide the corresponding dialkyl diazene-1,2-dicarboxylates, which can be reused in the cycloaddition reactions.
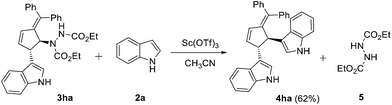 | ||
| Scheme 4 Lewis acid catalyzed synthesis of bisindole derivative catalyzed synthesis of bisindole derivative. | ||
In summary, we have developed a Lewis acid catalyzed C-3 alkylideneclopentenylation of indoles through the ring opening of pentafulvene derived diazabicyclic olefins. The developed method provides an efficient synthetic route to furnish pharmaceutically valuable indole and bisindole derivatives of alkylidenecyclopentenes from easily accessible starting materials. While multiple steps are involved in conventional synthetic strategies, this protocol offers a one-pot access to cyclopentene–bisindole hybrids. Moreover, the present strategy is compatible with both N-alkyl and free (NH) indoles. Further investigations to elaborate the scope of the reaction on other N-heterocycles and also to explore the biological applications of synthesized molecules are currently underway.
Experimental section
General methods
All chemicals were of the best grade commercially available and are used without further purification. All solvents were purified according to standard procedure; dry solvents were obtained according to the literature methods and stored over molecular sieves. Analytical thin layer chromatography was performed on glass plates coated with silica gel containing calcium sulfate binder. Gravity column chromatography was performed using 60–120 or 100–200 mesh silica gel and mixtures of hexane/ethyl acetate were used for elution.Melting points were determined on a Buchi melting point apparatus and are uncorrected. Proton nuclear magnetic resonance spectra (1H NMR) were recorded on a Bruker AMX 500 spectrophotometer (CDCl3 as solvent). Chemical shifts for 1H NMR spectra are reported as δ in units of parts per million (ppm) downfield from SiMe4 (δ 0.0) and relative to the signal of chloroform-d (δ 7.25, singlet). Multiplicities were given as: s (singlet); d (doublet); t (triplet); q (quadret); dd (double doublet); m (multiplet). Coupling constants are reported as J value in Hz. Carbon nuclear magnetic resonance spectra (13C NMR) are reported as δ in units of parts per million (ppm) downfield from SiMe4 (δ 0.0) and relative to the signal of chloroform-d (δ 77.03, triplet). Mass spectra were recorded under EI/HRMS at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 resolution using Thermo Scientific Exactive mass spectrometer. IR spectra were recorded on Bruker FT-IR spectrometer.
000 resolution using Thermo Scientific Exactive mass spectrometer. IR spectra were recorded on Bruker FT-IR spectrometer.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3334, 3054, 2976, 2920, 2853, 1709, 1586, 1458, 1410, 1330, 1220, 1120, 1052, 920, 745 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.10 (brs, 1H), 7.68 (brs, .03) (m, 1H), 6.84 (s, 1H), 6.53 (d, J = 6 Hz, 1H), 6.26 (brs, 1H), 6.04 (brs, 1H), 5.34–5.12 (m, 1H), 4.50–4.40 (m, 1H), 4.24–4.17 (m, 4H), 2.39–2.33 (m, 2H), 2.08–2.07 (m, 2H), 1.66–1.53 (m, 6H), 1.30–1.29 (m, 5H), 1.02 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.6, 155.1, 136.7, 136.2, 134.0, 129.7, 126.6, 121.9, 120.0, 119.2, 118.1, 110.0, 65.4, 64.1, 62.4, 61.9, 47.4, 32.0, 31.0, 28.4, 28.1, 26.6, 14.5, 14.2. HRMS (ESI): calcd for C25H31N3O4Na: 460.22123; found: 460.22171.
1). IR (Neat) νmax: 3334, 3054, 2976, 2920, 2853, 1709, 1586, 1458, 1410, 1330, 1220, 1120, 1052, 920, 745 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.10 (brs, 1H), 7.68 (brs, .03) (m, 1H), 6.84 (s, 1H), 6.53 (d, J = 6 Hz, 1H), 6.26 (brs, 1H), 6.04 (brs, 1H), 5.34–5.12 (m, 1H), 4.50–4.40 (m, 1H), 4.24–4.17 (m, 4H), 2.39–2.33 (m, 2H), 2.08–2.07 (m, 2H), 1.66–1.53 (m, 6H), 1.30–1.29 (m, 5H), 1.02 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.6, 155.1, 136.7, 136.2, 134.0, 129.7, 126.6, 121.9, 120.0, 119.2, 118.1, 110.0, 65.4, 64.1, 62.4, 61.9, 47.4, 32.0, 31.0, 28.4, 28.1, 26.6, 14.5, 14.2. HRMS (ESI): calcd for C25H31N3O4Na: 460.22123; found: 460.22171.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3323, 3055, 2981, 2932, 2855, 1710, 1619, 1583, 1513, 1458, 1415, 1339, 1302, 1227, 1096, 1061, 920, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.68 (brs, 1H), 7.25–7.23 (m, 2H), 7.05 (t, J = 7 Hz, 1H), 6.73 (s, 1H), 6.53 (d, J = 5.5, 1H), 6.23 (brs, 1H), 6.04 (s, 1H), 5.31–5.09 (m, 1H), 4.49–4.39 (m, 1H), 4.24–4.18 (m, 4H), 3.72 (s, 3H), 2.38–2.34 (m, 2H), 2.07–2.03 (m, 2H), 1.61–1.53 (m, 6H), 1.31–1.26 (m, 5H), 1.05–1.04 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.4, 155.1, 137.4, 133.7, 129.6, 127.0, 125.7, 121.5, 120.1, 118.7, 109.1, 108.8, 65.5, 62.3, 61.8, 47.5, 32.5, 31.9, 28.3, 28.0, 26.5, 14.5. HRMS (ESI): calcd for C26H33N3O4Na: 474.23688; found: 474.23764.
1). IR (Neat) νmax: 3323, 3055, 2981, 2932, 2855, 1710, 1619, 1583, 1513, 1458, 1415, 1339, 1302, 1227, 1096, 1061, 920, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.68 (brs, 1H), 7.25–7.23 (m, 2H), 7.05 (t, J = 7 Hz, 1H), 6.73 (s, 1H), 6.53 (d, J = 5.5, 1H), 6.23 (brs, 1H), 6.04 (s, 1H), 5.31–5.09 (m, 1H), 4.49–4.39 (m, 1H), 4.24–4.18 (m, 4H), 3.72 (s, 3H), 2.38–2.34 (m, 2H), 2.07–2.03 (m, 2H), 1.61–1.53 (m, 6H), 1.31–1.26 (m, 5H), 1.05–1.04 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.4, 155.1, 137.4, 133.7, 129.6, 127.0, 125.7, 121.5, 120.1, 118.7, 109.1, 108.8, 65.5, 62.3, 61.8, 47.5, 32.5, 31.9, 28.3, 28.0, 26.5, 14.5. HRMS (ESI): calcd for C26H33N3O4Na: 474.23688; found: 474.23764.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3324, 2980, 2930, 2854, 1701, 1519, 1472, 1420, 1382, 1332, 1261, 1233, 1097, 1060 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.16 (brs, 1H), 7.60–7.25 (m, 6H), 7.19–7.03 (m, 2H), 7.03 (d, J = 7 Hz, 1H), 6.55 (brs, 1H), 6.20–6.03 (m, 1H), 5.91 (brs, 1H), 5.59–5.45 (m, 1H), 4.68–4.53 (m, 1H), 4.16–4.12 (m, 4H), 2.58 (brs, 1H), 2.39–2.12 (m, 3H), 1.75–1.59 (m, 6H), 1.29–0.88 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.2, 155.4, 137.7, 136.3, 134.7, 132.8, 129.0, 128.6, 128.2, 127.6, 125.3, 121.9, 120.3, 119.4, 110.9, 62.4, 61.7, 60.3, 48.3, 34.6, 32.1, 26.9, 26.7, 21.5, 14.5, 14.2. HRMS (ESI): calcd for C31H35N3O4Na: 536.25253; found: 536.25289.
1). IR (Neat) νmax: 3324, 2980, 2930, 2854, 1701, 1519, 1472, 1420, 1382, 1332, 1261, 1233, 1097, 1060 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.16 (brs, 1H), 7.60–7.25 (m, 6H), 7.19–7.03 (m, 2H), 7.03 (d, J = 7 Hz, 1H), 6.55 (brs, 1H), 6.20–6.03 (m, 1H), 5.91 (brs, 1H), 5.59–5.45 (m, 1H), 4.68–4.53 (m, 1H), 4.16–4.12 (m, 4H), 2.58 (brs, 1H), 2.39–2.12 (m, 3H), 1.75–1.59 (m, 6H), 1.29–0.88 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.2, 155.4, 137.7, 136.3, 134.7, 132.8, 129.0, 128.6, 128.2, 127.6, 125.3, 121.9, 120.3, 119.4, 110.9, 62.4, 61.7, 60.3, 48.3, 34.6, 32.1, 26.9, 26.7, 21.5, 14.5, 14.2. HRMS (ESI): calcd for C31H35N3O4Na: 536.25253; found: 536.25289.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3363, 3277, 3054, 2984, 2931, 2854, 1711, 1582, 1500, 1149, 1411, 1330, 1120, 1050, 1010, 919, 744 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.26 (s, 1H), 7.34 (brs, 1H), 7.27–7.22 (m, 1H), 6.96–6.92 (brs, 1H), 6.65–6.56 (m, 2H), 6.40–6.31 (m, 1H), 6.02 (d, J = 3.5 Hz, 1H), 5.32–5.11 (m, 1H), 4.46–4.18 (m, 5H), 2.41–2.33 (m, 2H), 2.07–2.05 (m, 2H), 1.62–1.45 (m, 6H), 1.35–1.07 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 158.5, 156.8, 155.3, 136.9, 133.3, 130.0, 126.9, 123.1, 118.1, 111.5, 110.3, 104.9, 65.4, 62.6, 62.3, 47.5, 32.0, 31.1, 28.3, 28.0, 26.5, 14.4. HRMS (ESI): calcd for C25H30FN3O4Na: 478.21180; found: 478.21223.
1). IR (Neat) νmax: 3363, 3277, 3054, 2984, 2931, 2854, 1711, 1582, 1500, 1149, 1411, 1330, 1120, 1050, 1010, 919, 744 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.26 (s, 1H), 7.34 (brs, 1H), 7.27–7.22 (m, 1H), 6.96–6.92 (brs, 1H), 6.65–6.56 (m, 2H), 6.40–6.31 (m, 1H), 6.02 (d, J = 3.5 Hz, 1H), 5.32–5.11 (m, 1H), 4.46–4.18 (m, 5H), 2.41–2.33 (m, 2H), 2.07–2.05 (m, 2H), 1.62–1.45 (m, 6H), 1.35–1.07 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 158.5, 156.8, 155.3, 136.9, 133.3, 130.0, 126.9, 123.1, 118.1, 111.5, 110.3, 104.9, 65.4, 62.6, 62.3, 47.5, 32.0, 31.1, 28.3, 28.0, 26.5, 14.4. HRMS (ESI): calcd for C25H30FN3O4Na: 478.21180; found: 478.21223.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3365, 3071, 2960, 2852, 1712, 1623, 1582, 1469, 1410, 1380, 1318, 1245, 1173, 1115, 1058, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 9.22 (brs, 1H), 8.56 (s, 1H), 7.92 (brs, 1H), 7.17–7.13 (m, 1H), 6.92–6.82 (m, 1H), 6.61 (d, 1H, J = 4.5 Hz), 6.34 (brs, 1H), 5.98 (brs, 1H), 5.39–5.17 (m, 1H), 4.49–4.23 (m, 5H), 2.56–2.06 (m, 4H), 1.76–1.22 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.3, 155.6, 141.3, 139.8, 137.7, 130.1, 129.0, 128.2, 125.5, 125.3, 124.2, 117.6, 117.2, 111.0, 64.3, 62.9, 62.2, 47.5, 32.1, 31.3, 28.2, 26.6, 21.5, 14.5, 14.2. HRMS (ESI): calcd for C25H30N4O6Na: 505.20630; found: 505.20668.
1). IR (Neat) νmax: 3365, 3071, 2960, 2852, 1712, 1623, 1582, 1469, 1410, 1380, 1318, 1245, 1173, 1115, 1058, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 9.22 (brs, 1H), 8.56 (s, 1H), 7.92 (brs, 1H), 7.17–7.13 (m, 1H), 6.92–6.82 (m, 1H), 6.61 (d, 1H, J = 4.5 Hz), 6.34 (brs, 1H), 5.98 (brs, 1H), 5.39–5.17 (m, 1H), 4.49–4.23 (m, 5H), 2.56–2.06 (m, 4H), 1.76–1.22 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.3, 155.6, 141.3, 139.8, 137.7, 130.1, 129.0, 128.2, 125.5, 125.3, 124.2, 117.6, 117.2, 111.0, 64.3, 62.9, 62.2, 47.5, 32.1, 31.3, 28.2, 26.6, 21.5, 14.5, 14.2. HRMS (ESI): calcd for C25H30N4O6Na: 505.20630; found: 505.20668.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3380, 3280, 3054, 2976, 2928, 2853, 1709, 1586, 1499, 1149, 1410, 1330, 1220, 1120, 1052, 1011, 920, 745 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.89 (brs, 1H), 7.23–7.15 (m, 2H), 6.79–6.77 (m, 1H), 6.56–6.28 (m, 2H), 6.05 (brs, 1H), 5.32–5.09 (m, 1H), 4.45–4.11 (m, 5H), 2.37–2.33 (m, 2H), 2.07–2.06 (m, 2H), 1.60–1.38 (m, 6H), 1.29–1.13 (m, 5H), 0.99 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 155.2, 154.5, 147.5, 135.8, 135.0, 134.3, 127.2, 126.8, 125.3, 111.9, 111.8, 108.5, 104.5, 64.9, 62.8, 62.2, 41.9, 32.0, 28.2, 26.5, 19.4, 19.2, 14.5. HRMS (ESI): calcd for C25H31N3O5Na: 476.21614; found: 476.21658.
1). IR (Neat) νmax: 3380, 3280, 3054, 2976, 2928, 2853, 1709, 1586, 1499, 1149, 1410, 1330, 1220, 1120, 1052, 1011, 920, 745 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.89 (brs, 1H), 7.23–7.15 (m, 2H), 6.79–6.77 (m, 1H), 6.56–6.28 (m, 2H), 6.05 (brs, 1H), 5.32–5.09 (m, 1H), 4.45–4.11 (m, 5H), 2.37–2.33 (m, 2H), 2.07–2.06 (m, 2H), 1.60–1.38 (m, 6H), 1.29–1.13 (m, 5H), 0.99 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 155.2, 154.5, 147.5, 135.8, 135.0, 134.3, 127.2, 126.8, 125.3, 111.9, 111.8, 108.5, 104.5, 64.9, 62.8, 62.2, 41.9, 32.0, 28.2, 26.5, 19.4, 19.2, 14.5. HRMS (ESI): calcd for C25H31N3O5Na: 476.21614; found: 476.21658.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3331, 3068, 2981, 2932, 2857, 1688, 1621, 1583, 1514, 1462, 1380, 1304, 1238, 1108, 1042, 957, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.16 (brs, 1H), 7.75–7.71 (m, 1H), 7.31–7.23 (m, 1H), 7.18–7.05 (m, 2H), 6.88 (brs, 1H), 6.56–6.27 (m, 2H), 6.07 (brs, 1H), 5.34–5.14 (m, 1H), 5.00–4.95 (m, 2H), 4.53–4.43 (m, 1H), 2.36 (brs, 2H), 2.09–1.81 (m, 2H), 1.61–1.51 (m, 6H), 1.44–1.22 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.5, 154.7, 136.8, 133.8, 129.8, 129.0, 128.2, 126.7, 125.3, 121.7, 119.1, 110.9, 69.9, 69.5, 63.9, 47.2, 31.6, 30.8, 29.7, 28.3, 26.9, 22.7, 22.4, 22.1. HRMS (ESI): calcd for C27H35N3O5Na: 488.25253; found: 488.25286.
1). IR (Neat) νmax: 3331, 3068, 2981, 2932, 2857, 1688, 1621, 1583, 1514, 1462, 1380, 1304, 1238, 1108, 1042, 957, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.16 (brs, 1H), 7.75–7.71 (m, 1H), 7.31–7.23 (m, 1H), 7.18–7.05 (m, 2H), 6.88 (brs, 1H), 6.56–6.27 (m, 2H), 6.07 (brs, 1H), 5.34–5.14 (m, 1H), 5.00–4.95 (m, 2H), 4.53–4.43 (m, 1H), 2.36 (brs, 2H), 2.09–1.81 (m, 2H), 1.61–1.51 (m, 6H), 1.44–1.22 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.5, 154.7, 136.8, 133.8, 129.8, 129.0, 128.2, 126.7, 125.3, 121.7, 119.1, 110.9, 69.9, 69.5, 63.9, 47.2, 31.6, 30.8, 29.7, 28.3, 26.9, 22.7, 22.4, 22.1. HRMS (ESI): calcd for C27H35N3O5Na: 488.25253; found: 488.25286.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3375, 3078, 2992, 2943, 2836, 1690, 1610, 1583, 1565, 1468, 1462, 1400, 1316, 1238, 1152, 1123, 969, 938, 746 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.00 (d, J = 11 Hz, 1H), 7.99–7.79 (m, 1H), 7.32–7.28 (m, 1H), 7.20–7.06 (m, 2H), 6.87 (s, 1H), 6.55 (d, J = 5.5 Hz, 1H), 6.15–6.00 (m, 2H), 5.30–5.08 (m, 1H), 4.54–4.44 (m, 1H), 2.37 (brs, 2H), 2.12 (brs, 2H), 1.63–1.53 (m, 24H). 13C NMR (125 MHz, CDCl3, TMS): δ 155.7, 154.0, 136.7, 136.5, 126.7, 122.1, 121.8, 119.2, 118.2, 111.1, 110.9, 110.7, 81.3, 80.7, 65.5, 44.3, 32.0, 31.1, 28.3, 28.2, 28.0, 26.6. HRMS (ESI): calcd for C29H39N3O4Na: 516.28383; found: 516.28414.
1). IR (Neat) νmax: 3375, 3078, 2992, 2943, 2836, 1690, 1610, 1583, 1565, 1468, 1462, 1400, 1316, 1238, 1152, 1123, 969, 938, 746 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.00 (d, J = 11 Hz, 1H), 7.99–7.79 (m, 1H), 7.32–7.28 (m, 1H), 7.20–7.06 (m, 2H), 6.87 (s, 1H), 6.55 (d, J = 5.5 Hz, 1H), 6.15–6.00 (m, 2H), 5.30–5.08 (m, 1H), 4.54–4.44 (m, 1H), 2.37 (brs, 2H), 2.12 (brs, 2H), 1.63–1.53 (m, 24H). 13C NMR (125 MHz, CDCl3, TMS): δ 155.7, 154.0, 136.7, 136.5, 126.7, 122.1, 121.8, 119.2, 118.2, 111.1, 110.9, 110.7, 81.3, 80.7, 65.5, 44.3, 32.0, 31.1, 28.3, 28.2, 28.0, 26.6. HRMS (ESI): calcd for C29H39N3O4Na: 516.28383; found: 516.28414.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3358, 3059, 3027, 2920, 2858, 1702, 1580, 1489, 1449, 1400, 1311, 1281, 1050, 1000, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.28 (brs, 1H), 7.66 (brs, 1H), 7.39–6.90 (m, 13H), 6.75 (brs, 2H), 6.46 (s, 1H), 5.98–5.86 (m, 1H), 5.36–5.05 (m, 5H), 4.52–4.29 (m, 1H), 2.36–2.32 (m, 2H), 2.02–1.94 (m, 2H), 1.56–1.26 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.4, 154.8, 136.7, 135.8, 133.4, 128.6, 128.5, 128.3, 128.2, 127.9, 126.6, 122.0, 121.2, 119.9, 119.4, 117.5, 110.9, 68.1, 67.6, 47.5, 32.0, 31.0, 28.3, 28.0, 26.5. HRMS (ESI): calcd for C35H35N3O4Na: 584.25253; found: 584.25288.
1). IR (Neat) νmax: 3358, 3059, 3027, 2920, 2858, 1702, 1580, 1489, 1449, 1400, 1311, 1281, 1050, 1000, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.28 (brs, 1H), 7.66 (brs, 1H), 7.39–6.90 (m, 13H), 6.75 (brs, 2H), 6.46 (s, 1H), 5.98–5.86 (m, 1H), 5.36–5.05 (m, 5H), 4.52–4.29 (m, 1H), 2.36–2.32 (m, 2H), 2.02–1.94 (m, 2H), 1.56–1.26 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.4, 154.8, 136.7, 135.8, 133.4, 128.6, 128.5, 128.3, 128.2, 127.9, 126.6, 122.0, 121.2, 119.9, 119.4, 117.5, 110.9, 68.1, 67.6, 47.5, 32.0, 31.0, 28.3, 28.0, 26.5. HRMS (ESI): calcd for C35H35N3O4Na: 584.25253; found: 584.25288.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3317, 3056, 2982, 2931, 1719, 1620, 1582, 1512, 1415, 1382, 1229, 1096, 1062, 744 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.30 (s, 1H), 7.71 (brs, 1H), 7.34–7.27 (m, 1H), 7.19–7.08 (m, 2H), 6.86–6.78 (m, 2H), 6.52 (d, 1H, J = 5 Hz), 6.05 (s, 1H), 5.35–5.14 (m, 1H), 4.53–4.18 (m, 5H), 1.89 (s, 3H), 1.67 (brs, 3H), 1.29–1.26 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.9, 155.8, 136.8, 136.6, 135.5, 129.0, 126.7, 125.3, 121.7, 119.9, 119.1, 119.0, 117.9, 111.3, 66.0, 62.6, 62.2, 47.6, 21.5, 14.4. HRMS (ESI): calcd for C22H27N3O4Na: 420.18993; found: 420.18866.
1). IR (Neat) νmax: 3317, 3056, 2982, 2931, 1719, 1620, 1582, 1512, 1415, 1382, 1229, 1096, 1062, 744 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.30 (s, 1H), 7.71 (brs, 1H), 7.34–7.27 (m, 1H), 7.19–7.08 (m, 2H), 6.86–6.78 (m, 2H), 6.52 (d, 1H, J = 5 Hz), 6.05 (s, 1H), 5.35–5.14 (m, 1H), 4.53–4.18 (m, 5H), 1.89 (s, 3H), 1.67 (brs, 3H), 1.29–1.26 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.9, 155.8, 136.8, 136.6, 135.5, 129.0, 126.7, 125.3, 121.7, 119.9, 119.1, 119.0, 117.9, 111.3, 66.0, 62.6, 62.2, 47.6, 21.5, 14.4. HRMS (ESI): calcd for C22H27N3O4Na: 420.18993; found: 420.18866.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3385, 3055, 2981, 2924, 1707, 1611, 1474, 1413, 1379, 1321, 1265, 1219, 1163, 1122, 1061, 1021, 933, 739 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.73 (s, 1H), 7.29–7.23 (m, 2H), 7.10 (t, J = 7 Hz, 1H), 6.77 (brs, 1H), 6.53 (d, J = 5 Hz, 1H), 6.39 (brs, 1H), 6.07 (s, 1H), 5.36–5.14 (m, 1H), 4.53–4.20 (m, 5H), 3.73 (s, 3H), 1.90 (s, 3H), 1.69 (s, 3H), 1.31–1.05 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.7, 155.6, 137.5, 136.7, 135.6, 130.5, 128.3, 127.1, 125.9, 121.6, 120.1, 118.8, 116.7, 109.0, 66.2, 62.5, 61.9, 47.5, 32.6, 21.5, 13.8. HRMS (ESI): calcd for C23H29N3O4Na: 434.20588; found: 434.20615.
1). IR (Neat) νmax: 3385, 3055, 2981, 2924, 1707, 1611, 1474, 1413, 1379, 1321, 1265, 1219, 1163, 1122, 1061, 1021, 933, 739 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.73 (s, 1H), 7.29–7.23 (m, 2H), 7.10 (t, J = 7 Hz, 1H), 6.77 (brs, 1H), 6.53 (d, J = 5 Hz, 1H), 6.39 (brs, 1H), 6.07 (s, 1H), 5.36–5.14 (m, 1H), 4.53–4.20 (m, 5H), 3.73 (s, 3H), 1.90 (s, 3H), 1.69 (s, 3H), 1.31–1.05 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.7, 155.6, 137.5, 136.7, 135.6, 130.5, 128.3, 127.1, 125.9, 121.6, 120.1, 118.8, 116.7, 109.0, 66.2, 62.5, 61.9, 47.5, 32.6, 21.5, 13.8. HRMS (ESI): calcd for C23H29N3O4Na: 434.20588; found: 434.20615.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3348, 3056, 2924, 2853, 1708, 1617, 1458, 1414, 1380, 1226, 1177, 1121, 1061, 741 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.09 (brs, 1H), 7.66 (brs, 1H), 7.28 (brs, 1H), 7.16–7.04 (m 2H), 6.84 (brs, 1H), 6.51 (d, J = 5.5 Hz, 1H), 6.25–6.21 (m, 1H), 6.04 (brs, 1H), 5.33–5.11 (m, 1H), 4.50–4.18 (m, 5H), 2.50–2.41 (m, 2H), 2.20–2.16 (brs, 2H), 1.71–1.03 (m, 14H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.3, 155.5, 136.8, 129.0, 128.2, 126.6, 125.3, 121.7, 119.0, 119.0, 111.1, 62.4, 61.9, 47.6, 32.7, 32.3, 29.1, 28.2, 27.6, 14.5, 14.2. HRMS (ESI): calcd for C26H33N3O4Na: 474.23688; found: 474.23714.
1). IR (Neat) νmax: 3348, 3056, 2924, 2853, 1708, 1617, 1458, 1414, 1380, 1226, 1177, 1121, 1061, 741 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.09 (brs, 1H), 7.66 (brs, 1H), 7.28 (brs, 1H), 7.16–7.04 (m 2H), 6.84 (brs, 1H), 6.51 (d, J = 5.5 Hz, 1H), 6.25–6.21 (m, 1H), 6.04 (brs, 1H), 5.33–5.11 (m, 1H), 4.50–4.18 (m, 5H), 2.50–2.41 (m, 2H), 2.20–2.16 (brs, 2H), 1.71–1.03 (m, 14H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.3, 155.5, 136.8, 129.0, 128.2, 126.6, 125.3, 121.7, 119.0, 119.0, 111.1, 62.4, 61.9, 47.6, 32.7, 32.3, 29.1, 28.2, 27.6, 14.5, 14.2. HRMS (ESI): calcd for C26H33N3O4Na: 474.23688; found: 474.23714.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3323, 3057, 2920, 2848, 1713, 1620, 1475, 1413, 1381, 1305, 1294, 1216, 1116, 1085, 1065, 1025, 742 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.21 (brs, 1H), 7.70 (brs, 1H), 7.32–7.25 (m, 2H), 7.20–7.05 (m, 3H), 6.86 (brs, 1H), 6.56 (d, J = 5.5 Hz, 1H), 6.29 (brs, 1H), 6.05 (brs, 1H), 5.39–5.16 (m, 1H), 4.53–4.41 (m, 1H), 4.30–4.13 (m, 4H), 3.06 (brs, 1H), 2.59 (brs, 1H), 2.08–1.64 (m, 12H), 1.35–1.08 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.7, 155.2, 144.4, 136.5, 130.2, 129.0, 128.3, 126.6, 125.3, 121.4, 121.1, 119.9, 119.0, 117.1, 111.1, 63.8, 62.5, 62.0, 47.6, 39.9, 39.5, 39.1, 37.0, 35.1, 34.4, 28.1, 28.0, 21.5, 14.6. HRMS (ESI): calcd for C29H35N3O4: 512.25253; found: 515.25290.
1). IR (Neat) νmax: 3323, 3057, 2920, 2848, 1713, 1620, 1475, 1413, 1381, 1305, 1294, 1216, 1116, 1085, 1065, 1025, 742 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.21 (brs, 1H), 7.70 (brs, 1H), 7.32–7.25 (m, 2H), 7.20–7.05 (m, 3H), 6.86 (brs, 1H), 6.56 (d, J = 5.5 Hz, 1H), 6.29 (brs, 1H), 6.05 (brs, 1H), 5.39–5.16 (m, 1H), 4.53–4.41 (m, 1H), 4.30–4.13 (m, 4H), 3.06 (brs, 1H), 2.59 (brs, 1H), 2.08–1.64 (m, 12H), 1.35–1.08 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.7, 155.2, 144.4, 136.5, 130.2, 129.0, 128.3, 126.6, 125.3, 121.4, 121.1, 119.9, 119.0, 117.1, 111.1, 63.8, 62.5, 62.0, 47.6, 39.9, 39.5, 39.1, 37.0, 35.1, 34.4, 28.1, 28.0, 21.5, 14.6. HRMS (ESI): calcd for C29H35N3O4: 512.25253; found: 515.25290.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3315, 3054, 2910, 2852, 1711, 1612, 1472, 1413, 1379, 1305, 1221, 1124, 1061, 1019, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.71 (brs, 1H), 7.7–7.21 (m, 2H), 7.07 (t, J = 7 Hz, 1H), 6.80 (brs, 1H), 6.55 (d, J = 5.5 Hz, 1H), 6.25 (brs, 1H), 6.05 (s, 1H), 5.35–5.12 (m, 1H), 4.53–4.28 (m, 1H), 4.23–4.13 (m, 4H), 3.75 (s, 3H), 3.05 (s, 1H), 2.58 (brs, 1H), 2.02–1.63 (m, 12H), 1.37–1.09 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.6, 154.9, 137.4, 130.2, 127.1, 125.8, 121.5, 120.2, 118.7, 108.9, 62.3, 61.9, 47.2, 39.6, 37.0, 35.1, 34.7, 32.6, 28.1, 26.9, 25.3, 22.9, 20.8, 14.9. HRMS (ESI): calcd for C30H37N3O4Na: 526.26818; found: 526.26862.
1). IR (Neat) νmax: 3315, 3054, 2910, 2852, 1711, 1612, 1472, 1413, 1379, 1305, 1221, 1124, 1061, 1019, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.71 (brs, 1H), 7.7–7.21 (m, 2H), 7.07 (t, J = 7 Hz, 1H), 6.80 (brs, 1H), 6.55 (d, J = 5.5 Hz, 1H), 6.25 (brs, 1H), 6.05 (s, 1H), 5.35–5.12 (m, 1H), 4.53–4.28 (m, 1H), 4.23–4.13 (m, 4H), 3.75 (s, 3H), 3.05 (s, 1H), 2.58 (brs, 1H), 2.02–1.63 (m, 12H), 1.37–1.09 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.6, 154.9, 137.4, 130.2, 127.1, 125.8, 121.5, 120.2, 118.7, 108.9, 62.3, 61.9, 47.2, 39.6, 37.0, 35.1, 34.7, 32.6, 28.1, 26.9, 25.3, 22.9, 20.8, 14.9. HRMS (ESI): calcd for C30H37N3O4Na: 526.26818; found: 526.26862.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3378, 3058, 2978, 2908, 2848, 1756, 1704, 1467, 1445, 1409, 1379, 1364, 1338, 1308, 1277, 1248, 1218, 1172, 1157, 1097, 1062, 1022 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.58–7.44 (m, 6H), 7.33–7.19 (m, 3H), 7.04 (brs, 1H), 6.50–6.42 (m, 1H), 6.12–5.81 (m, 2H), 5.45 (brs, 1H), 4.25–4.15 (m, 4H), 3.58 (s, 3H), 3.06 (brs, 1H), 2.65–2.61 (m, 1H), 2.03–1.85 (m, 10H), 1.59–1.25 (m, 2H), 1.01–0.87 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.4, 155.3, 137.4, 131.3, 130.6, 128.1, 128.0, 121.5, 120.2, 119.0, 113.5, 109.3, 65.9, 62.3, 61.7, 47.8, 39.5, 39.4, 37.0, 35.1, 34.6, 30.8, 28.2, 28.1, 14.7. HRMS (ESI): calcd for C36H41N3O4Na: 602.29948; found: 602.29977.
1). IR (Neat) νmax: 3378, 3058, 2978, 2908, 2848, 1756, 1704, 1467, 1445, 1409, 1379, 1364, 1338, 1308, 1277, 1248, 1218, 1172, 1157, 1097, 1062, 1022 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.58–7.44 (m, 6H), 7.33–7.19 (m, 3H), 7.04 (brs, 1H), 6.50–6.42 (m, 1H), 6.12–5.81 (m, 2H), 5.45 (brs, 1H), 4.25–4.15 (m, 4H), 3.58 (s, 3H), 3.06 (brs, 1H), 2.65–2.61 (m, 1H), 2.03–1.85 (m, 10H), 1.59–1.25 (m, 2H), 1.01–0.87 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.4, 155.3, 137.4, 131.3, 130.6, 128.1, 128.0, 121.5, 120.2, 119.0, 113.5, 109.3, 65.9, 62.3, 61.7, 47.8, 39.5, 39.4, 37.0, 35.1, 34.6, 30.8, 28.2, 28.1, 14.7. HRMS (ESI): calcd for C36H41N3O4Na: 602.29948; found: 602.29977.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3362, 3051, 2968, 2911, 2852, 1736, 1710, 1552, 1514, 1467, 1454, 1411, 1384, 1364, 1308, 1287, 1243, 1231, 1168, 1157, 1069, 1063, 1022, 742 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.85–7.79 (m, 1H), 7.42–7.03 (m, 12H), 6.90–6.59 (m, 3H), 6.32–6.22 (brs, 1H), 6.04–5.91 (m, 2H), 5.08 (brs, 1H), 4.23–4.13 (m, 4H), 3.92–3.73 (m, 1H), 1.35–1.01 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.0, 154.7, 142.5, 142.4, 141.3, 140.8, 137.4, 130.0, 129.9, 128.6, 128.2, 127.7, 127.4, 126.8, 121.6, 120.2, 119.1, 116.9, 115.5, 110.2, 65.6, 62.0, 61.8, 47.9, 14.8. HRMS (ESI): calcd for C32H31N3O4Na: 544.22123; found: 544.22151.
1). IR (Neat) νmax: 3362, 3051, 2968, 2911, 2852, 1736, 1710, 1552, 1514, 1467, 1454, 1411, 1384, 1364, 1308, 1287, 1243, 1231, 1168, 1157, 1069, 1063, 1022, 742 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.85–7.79 (m, 1H), 7.42–7.03 (m, 12H), 6.90–6.59 (m, 3H), 6.32–6.22 (brs, 1H), 6.04–5.91 (m, 2H), 5.08 (brs, 1H), 4.23–4.13 (m, 4H), 3.92–3.73 (m, 1H), 1.35–1.01 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.0, 154.7, 142.5, 142.4, 141.3, 140.8, 137.4, 130.0, 129.9, 128.6, 128.2, 127.7, 127.4, 126.8, 121.6, 120.2, 119.1, 116.9, 115.5, 110.2, 65.6, 62.0, 61.8, 47.9, 14.8. HRMS (ESI): calcd for C32H31N3O4Na: 544.22123; found: 544.22151.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3340, 3068, 2981, 2932, 2857, 1688, 1621, 1602, 1583, 1555, 1514, 1462, 1380, 1315, 1238, 1108, 1042, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.79 (brs, 1H), 7.34–7.19 (m, 14H), 7.09–6.97 (m, 2H), 6.61–6.55 (m, 1H), 6.32 (brs, 1H), 5.82–5.56 (m, 2H), 4.70–4.65 (m, 1H), 4.25–4.15 (m, 4H), 3.76 (brs, 3H), 1.32–1.29 (m, 4H), 1.03 (brs, 1H), 0.69 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.9, 154.9, 142.6, 142.3, 141.3, 140.7, 137.4, 130.0, 129.9, 128.5, 128.1, 127.4, 127.3, 127.1, 126.6, 121.4, 120.2, 118.8, 116.0, 115.3, 108.9, 65.5, 62.0, 61.8, 47.6, 32.6, 14.5, 13.8. HRMS (ESI): calcd for C33H33N3O4Na: 558.23688; found: 558.23721.
1). IR (Neat) νmax: 3340, 3068, 2981, 2932, 2857, 1688, 1621, 1602, 1583, 1555, 1514, 1462, 1380, 1315, 1238, 1108, 1042, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.79 (brs, 1H), 7.34–7.19 (m, 14H), 7.09–6.97 (m, 2H), 6.61–6.55 (m, 1H), 6.32 (brs, 1H), 5.82–5.56 (m, 2H), 4.70–4.65 (m, 1H), 4.25–4.15 (m, 4H), 3.76 (brs, 3H), 1.32–1.29 (m, 4H), 1.03 (brs, 1H), 0.69 (brs, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 156.9, 154.9, 142.6, 142.3, 141.3, 140.7, 137.4, 130.0, 129.9, 128.5, 128.1, 127.4, 127.3, 127.1, 126.6, 121.4, 120.2, 118.8, 116.0, 115.3, 108.9, 65.5, 62.0, 61.8, 47.6, 32.6, 14.5, 13.8. HRMS (ESI): calcd for C33H33N3O4Na: 558.23688; found: 558.23721.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3405, 2922, 2851, 2362, 2349, 1590, 1459, 1421, 1364, 1120, 1033 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.93 (s, 1H), 7.85 (s, 1H), 7.61–7.57 (m, 2H), 7.38–7.34 (m, 2H), 7.22–7.17 (m, 2H), 7.09–6.94 (m, 4H), 6.78 (d, J = 5.5 Hz, 1H), 6.04 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 4.32 (brs, 1H), 4.19 (brs, 1H), 2.46 (t, J = 6 Hz, 2H), 2.04–1.97 (m, 2H), 1.67–1.29 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 139.1, 136.9, 135.9, 133.0, 129.9, 129.1, 128.3, 126.7, 126.6, 125.4, 121.9, 121.8, 121.0, 120.9, 120.2, 120.1, 119.6, 118.9, 111.2, 111.0, 52.3, 45.8, 32.1, 31.8, 28.6, 27.7, 26.9. HRMS (ESI): calcd for C27H26N2Na: 401.19937; found: 401.19968.
1). IR (Neat) νmax: 3405, 2922, 2851, 2362, 2349, 1590, 1459, 1421, 1364, 1120, 1033 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.93 (s, 1H), 7.85 (s, 1H), 7.61–7.57 (m, 2H), 7.38–7.34 (m, 2H), 7.22–7.17 (m, 2H), 7.09–6.94 (m, 4H), 6.78 (d, J = 5.5 Hz, 1H), 6.04 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 4.32 (brs, 1H), 4.19 (brs, 1H), 2.46 (t, J = 6 Hz, 2H), 2.04–1.97 (m, 2H), 1.67–1.29 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 139.1, 136.9, 135.9, 133.0, 129.9, 129.1, 128.3, 126.7, 126.6, 125.4, 121.9, 121.8, 121.0, 120.9, 120.2, 120.1, 119.6, 118.9, 111.2, 111.0, 52.3, 45.8, 32.1, 31.8, 28.6, 27.7, 26.9. HRMS (ESI): calcd for C27H26N2Na: 401.19937; found: 401.19968.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 2935, 2855, 2358, 2353, 1680, 1595, 1449, 1431, 1358, 1156, 1120, 1033 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.56–7.52 (m, 2H), 7.28–7.16 (m, 4H), 7.14–6.99 (m, 2H), 6.83 (s, 1H), 6.75 (s, 1H), 6.71 (dd, J1 = 5.5 Hz, J2 = 1 Hz, 1H), 5.97 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 4.26 (s, 1H), 4.11 (s, 1H), 3.77 (s, 3H), 3.74 (s, 3H), 2.45–2.41 (m, 2H), 2.02–2.00 (m, 1H), 1.94–1.92 (m, 1H), 1.63–1.45 (m, 4H), 1.34–1.31 (m, 1H), 1.18–1.17 (m, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 139.2, 137.5, 137.4, 136.1, 132.8, 129.6, 129.0, 128.2, 127.1, 126.9, 125.6, 125.3, 121.5, 121.3, 120.4, 120.3, 120.2, 118.8, 118.6, 118.3, 109.1, 108.9, 52.3, 45.7, 32.6, 32.5, 32.0, 31.9, 28.6, 27.7, 26.9. HRMS (ESI): calcd for C29H30N2Na: 429.23067; found: 429.23102.
1). IR (Neat) νmax: 2935, 2855, 2358, 2353, 1680, 1595, 1449, 1431, 1358, 1156, 1120, 1033 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.56–7.52 (m, 2H), 7.28–7.16 (m, 4H), 7.14–6.99 (m, 2H), 6.83 (s, 1H), 6.75 (s, 1H), 6.71 (dd, J1 = 5.5 Hz, J2 = 1 Hz, 1H), 5.97 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 4.26 (s, 1H), 4.11 (s, 1H), 3.77 (s, 3H), 3.74 (s, 3H), 2.45–2.41 (m, 2H), 2.02–2.00 (m, 1H), 1.94–1.92 (m, 1H), 1.63–1.45 (m, 4H), 1.34–1.31 (m, 1H), 1.18–1.17 (m, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 139.2, 137.5, 137.4, 136.1, 132.8, 129.6, 129.0, 128.2, 127.1, 126.9, 125.6, 125.3, 121.5, 121.3, 120.4, 120.3, 120.2, 118.8, 118.6, 118.3, 109.1, 108.9, 52.3, 45.7, 32.6, 32.5, 32.0, 31.9, 28.6, 27.7, 26.9. HRMS (ESI): calcd for C29H30N2Na: 429.23067; found: 429.23102.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3342, 3075, 2953, 2912, 2857, 1695, 1611, 1514, 1462, 1380, 1238, 1100, 1030, 931, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.98 (s, 1H), 7.88 (s, 1H), 7.68 (d, J = 8 Hz, 1H), 7.59 (d, J = 8 Hz, 1H), 7.42–7.37 (m, 4H), 7.28–6.80 (m, 13H), 6.14 (m, 1H), 4.76 (brs, 1H), 4.71 (brs, 1H), 2.51–2.49 (m, 1H), 2.38–2.18 (m, 1H), 1.83–1.07 (m, 8H). 13C NMR (125 MHz, CDCl3, TMS): δ 138.4, 136.5, 136.4, 136.3, 135.1, 134.5, 133.4, 132.6, 132.5, 130.5, 128.4, 128.3, 128.2, 127.9, 127.8, 127.4, 127.3, 122.3, 122.2, 121.3, 120.9, 119.6, 119.2, 117.3, 114.7, 110.5, 110.3, 50.6, 44.8, 32.5, 30.8, 28.6, 27.1, 26.8. HRMS (ESI): calcd for C39H34N2Na: 553.26197; found: 553.26233.
1). IR (Neat) νmax: 3342, 3075, 2953, 2912, 2857, 1695, 1611, 1514, 1462, 1380, 1238, 1100, 1030, 931, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.98 (s, 1H), 7.88 (s, 1H), 7.68 (d, J = 8 Hz, 1H), 7.59 (d, J = 8 Hz, 1H), 7.42–7.37 (m, 4H), 7.28–6.80 (m, 13H), 6.14 (m, 1H), 4.76 (brs, 1H), 4.71 (brs, 1H), 2.51–2.49 (m, 1H), 2.38–2.18 (m, 1H), 1.83–1.07 (m, 8H). 13C NMR (125 MHz, CDCl3, TMS): δ 138.4, 136.5, 136.4, 136.3, 135.1, 134.5, 133.4, 132.6, 132.5, 130.5, 128.4, 128.3, 128.2, 127.9, 127.8, 127.4, 127.3, 122.3, 122.2, 121.3, 120.9, 119.6, 119.2, 117.3, 114.7, 110.5, 110.3, 50.6, 44.8, 32.5, 30.8, 28.6, 27.1, 26.8. HRMS (ESI): calcd for C39H34N2Na: 553.26197; found: 553.26233.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3356, 3052, 2978, 2939, 2849, 1689, 1619, 1583, 1514, 1462, 1415, 1402, 1380, 1304, 1238, 1111, 1047, 942, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.03 (s, 1H), 7.94 (s, 1H), 7.31–7.20 (m, 4H), 7.07 (s, 1H), 7.00–6.77 (m, 4H), 6.00 (t, 1H, J = 3 Hz), 4.22 (s, 1H), 4.09 (s, 1H), 2.46–2.42 (m, 2H), 2.06–2.04 (m, 1H), 1.96–1.94 (m, 1H), 1.67–1.44 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 158.5, 156.7, 138.5, 135.3, 133.8, 133.5, 130.3, 126.9, 122.9, 122.7, 121.9, 120.3, 111.7, 111.6, 110.5, 110.3, 110.2, 105.2, 105.0, 45.6, 32.0, 31.8, 28.5, 27.6, 26.8. HRMS (ESI): calcd for C27H24F2N2Na: 437.18052; found: 437.18088.
1). IR (Neat) νmax: 3356, 3052, 2978, 2939, 2849, 1689, 1619, 1583, 1514, 1462, 1415, 1402, 1380, 1304, 1238, 1111, 1047, 942, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.03 (s, 1H), 7.94 (s, 1H), 7.31–7.20 (m, 4H), 7.07 (s, 1H), 7.00–6.77 (m, 4H), 6.00 (t, 1H, J = 3 Hz), 4.22 (s, 1H), 4.09 (s, 1H), 2.46–2.42 (m, 2H), 2.06–2.04 (m, 1H), 1.96–1.94 (m, 1H), 1.67–1.44 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 158.5, 156.7, 138.5, 135.3, 133.8, 133.5, 130.3, 126.9, 122.9, 122.7, 121.9, 120.3, 111.7, 111.6, 110.5, 110.3, 110.2, 105.2, 105.0, 45.6, 32.0, 31.8, 28.5, 27.6, 26.8. HRMS (ESI): calcd for C27H24F2N2Na: 437.18052; found: 437.18088.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3326, 3056, 2955, 2932, 2850, 1675, 1629, 1583, 1457, 1385, 1300, 1238, 1100, 1040, 931, 7445 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.91 (s, 1H), 8.74 (s, 1H), 8.53–8.52 (m, 2H), 8.12–8.09 (m, 2H), 7.45–7.41 (m, 2H), 7.26 (d, J = 10.5 Hz, 1H), 7.14 (s, 1H), 6.86 (d, J = 5.5 Hz, 1H), 5.99 (d, J = 4.5 Hz, 1H), 4.33 (s, 1H), 4.22 (brs, 1H), 2.61–2.58 (m, 1H), 2.44–2.42 (m, 1H), 2.07–2.04 (m, 1H), 1.93–1.90 (m, 1H), 1.89–1.37 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 141.4, 141.2, 140.1, 140.0, 137.6, 135.1, 134.6, 130.9, 125.9, 125.7, 124.1, 124.0, 123.8, 122.2, 117.7, 117.6, 117.5, 112.9, 111.3, 52.3, 45.7, 32.1, 32.0, 28.2, 27.7, 26.7. HRMS (ESI): calcd for C27H24N2O4Na: 491.16952; found: 491.16993.
1). IR (Neat) νmax: 3326, 3056, 2955, 2932, 2850, 1675, 1629, 1583, 1457, 1385, 1300, 1238, 1100, 1040, 931, 7445 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 8.91 (s, 1H), 8.74 (s, 1H), 8.53–8.52 (m, 2H), 8.12–8.09 (m, 2H), 7.45–7.41 (m, 2H), 7.26 (d, J = 10.5 Hz, 1H), 7.14 (s, 1H), 6.86 (d, J = 5.5 Hz, 1H), 5.99 (d, J = 4.5 Hz, 1H), 4.33 (s, 1H), 4.22 (brs, 1H), 2.61–2.58 (m, 1H), 2.44–2.42 (m, 1H), 2.07–2.04 (m, 1H), 1.93–1.90 (m, 1H), 1.89–1.37 (m, 6H). 13C NMR (125 MHz, CDCl3, TMS): δ 141.4, 141.2, 140.1, 140.0, 137.6, 135.1, 134.6, 130.9, 125.9, 125.7, 124.1, 124.0, 123.8, 122.2, 117.7, 117.6, 117.5, 112.9, 111.3, 52.3, 45.7, 32.1, 32.0, 28.2, 27.7, 26.7. HRMS (ESI): calcd for C27H24N2O4Na: 491.16952; found: 491.16993.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3339, 3061, 2990, 2940, 2842, 1680, 1623, 1580, 1514, 1380, 1302, 1240, 1110, 1042, 931, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.87 (brs, 1H), 7.78 (brs, 1H), 7.27–7.22 (m, 2H), 7.03–6.98 (m, 3H), 6.92 (d J = 2 Hz, 1H), 6.81–6.74 (m 3H), 6.01–6.00 (dd, J1 = 6 Hz, J2 = 3 Hz, 1H), 4.82 (d, J = 6.5 Hz, 2H), 4.16 (s, 1H), 4.07 (s, 1H), 2.45–2.39 (m, 2H), 1.99–1.94 (m, 2H), 1.50–1.44 (m 3H), 1.33–0.87 (m 5H). 13C NMR (125 MHz, CDCl3, TMS): δ 149.1, 148.9, 138.8, 135.6, 133.1, 132.1, 130.0, 127.3, 122.2, 121.3, 119.8, 111.8, 111.7, 111.6, 111.6, 104.8, 104.7, 51.7, 45.8, 32.0, 31.8, 28.6, 27.6, 26.8. HRMS (ESI): calcd for C27H26N2O2Na: 433.18920; found: 433.18954.
1). IR (Neat) νmax: 3339, 3061, 2990, 2940, 2842, 1680, 1623, 1580, 1514, 1380, 1302, 1240, 1110, 1042, 931, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.87 (brs, 1H), 7.78 (brs, 1H), 7.27–7.22 (m, 2H), 7.03–6.98 (m, 3H), 6.92 (d J = 2 Hz, 1H), 6.81–6.74 (m 3H), 6.01–6.00 (dd, J1 = 6 Hz, J2 = 3 Hz, 1H), 4.82 (d, J = 6.5 Hz, 2H), 4.16 (s, 1H), 4.07 (s, 1H), 2.45–2.39 (m, 2H), 1.99–1.94 (m, 2H), 1.50–1.44 (m 3H), 1.33–0.87 (m 5H). 13C NMR (125 MHz, CDCl3, TMS): δ 149.1, 148.9, 138.8, 135.6, 133.1, 132.1, 130.0, 127.3, 122.2, 121.3, 119.8, 111.8, 111.7, 111.6, 111.6, 104.8, 104.7, 51.7, 45.8, 32.0, 31.8, 28.6, 27.6, 26.8. HRMS (ESI): calcd for C27H26N2O2Na: 433.18920; found: 433.18954.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3315, 2920, 2857, 2377, 1648, 1590, 1520, 1468, 1367, 1160, 1119, 1037 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.99 (s, 1H), 7.92 (s, 1H), 7.60–7.56 (m, 2H), 7.40–7.37 (m, 2H), 7.23–7.18 (m, 3H), 7.09–7.05 (m, 3H), 7.00 (s, 1H), 6.94 (s, 1H), 6.74 (dd, 1H, J1 = 5.5 Hz, J2 = 2 Hz), 6.05 (dd, 1H, J1 = 5.5 Hz, J2 = 2.5 Hz), 4.28 (s, 1H), 4.22 (s, 1H), 1.93 (s, 3H), 1.61 (s, 3H). 13C NMR (125 MHz, CDCl3, TMS): δ 141.9, 137.1, 136.9, 135.8, 130.4, 127.3, 126.8, 125.8, 125.7, 124.4, 121.5, 121.3, 120.5, 120.3, 120.0, 118.4, 118.2, 117.9, 110.8, 110.7, 52.4, 46.4, 21.3. HRMS (ESI): calcd for C24H22N2Na: 361.16807; found: 361.16848.
1). IR (Neat) νmax: 3315, 2920, 2857, 2377, 1648, 1590, 1520, 1468, 1367, 1160, 1119, 1037 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.99 (s, 1H), 7.92 (s, 1H), 7.60–7.56 (m, 2H), 7.40–7.37 (m, 2H), 7.23–7.18 (m, 3H), 7.09–7.05 (m, 3H), 7.00 (s, 1H), 6.94 (s, 1H), 6.74 (dd, 1H, J1 = 5.5 Hz, J2 = 2 Hz), 6.05 (dd, 1H, J1 = 5.5 Hz, J2 = 2.5 Hz), 4.28 (s, 1H), 4.22 (s, 1H), 1.93 (s, 3H), 1.61 (s, 3H). 13C NMR (125 MHz, CDCl3, TMS): δ 141.9, 137.1, 136.9, 135.8, 130.4, 127.3, 126.8, 125.8, 125.7, 124.4, 121.5, 121.3, 120.5, 120.3, 120.0, 118.4, 118.2, 117.9, 110.8, 110.7, 52.4, 46.4, 21.3. HRMS (ESI): calcd for C24H22N2Na: 361.16807; found: 361.16848.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 2925, 2852, 2371, 1649, 1586, 1523, 1465, 1364, 1254, 1167, 1122, 1042 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.66–7.61 (m, 2H), 7.38–7.31 (m, 2H), 7.29–7.27 (m, 2H), 7.14–7.09 (m, 2H), 6.90 (s, 1H), 6.83 (s, 1H), 6.78 (dd, 1H, J1 = 5.5 Hz, J2 = 2 Hz), 6.09 (dd, H, J1 = 5.5 Hz, J2 = 2.5 Hz), 4.32 (s, 1H), 4.25 (s, 1H), 3.81 (s, 3H), 3.79 (s, 3H), 1.99 (s, 3H), 1.60 (s, 3H). 13C NMR (125 MHz, CDCl3, TMS): δ 142.2, 137.6, 137.5, 135.9, 130.5, 127.1, 127.0, 125.9, 125.7, 124.4, 121.5, 121.3, 120.3, 120.2, 119.9, 118.8, 118.7, 118.4, 109.2, 109.1, 52.6, 46.6, 31.7, 21.4. HRMS (ESI): calcd for C26H26N2Na: 389.19937; found: 389.19969.
1). IR (Neat) νmax: 2925, 2852, 2371, 1649, 1586, 1523, 1465, 1364, 1254, 1167, 1122, 1042 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.66–7.61 (m, 2H), 7.38–7.31 (m, 2H), 7.29–7.27 (m, 2H), 7.14–7.09 (m, 2H), 6.90 (s, 1H), 6.83 (s, 1H), 6.78 (dd, 1H, J1 = 5.5 Hz, J2 = 2 Hz), 6.09 (dd, H, J1 = 5.5 Hz, J2 = 2.5 Hz), 4.32 (s, 1H), 4.25 (s, 1H), 3.81 (s, 3H), 3.79 (s, 3H), 1.99 (s, 3H), 1.60 (s, 3H). 13C NMR (125 MHz, CDCl3, TMS): δ 142.2, 137.6, 137.5, 135.9, 130.5, 127.1, 127.0, 125.9, 125.7, 124.4, 121.5, 121.3, 120.3, 120.2, 119.9, 118.8, 118.7, 118.4, 109.2, 109.1, 52.6, 46.6, 31.7, 21.4. HRMS (ESI): calcd for C26H26N2Na: 389.19937; found: 389.19969.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3408, 3056, 2923, 2853, 1703, 1619, 1583, 1517, 1485, 1455, 1338, 1227, 1095, 1012, 741 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.97 (s, 1H), 7.89 (s, 1H), 7.59–7.57 (d, J = 8 Hz, 2H), 7.37–7.34 (m, 2H), 7.21–7.16 (m, 2H), 7.07–7.05 (m, 2H), 6.98–6.93 (m, 2H), 6.76 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 6.04 (dd, J1 = 5.5 Hz, J2 = 3 Hz, 1H), 4.27 (s, 1H), 4.18 (brs, 1H), 2.57–2.51 (m, 2H), 2.25–2.19 (m, 1H), 2.07–2.06 (m, 1H), 1.72–1.29 (m, 8H). 13C NMR (125 MHz, CDCl3, TMS): δ 142.1, 136.9, 136.7, 135.9, 134.3, 130.4, 129.0, 128.2, 126.7, 126.6, 121.9, 121.5, 120.8, 120.7, 120.2, 120.1, 119.2, 119.0, 111.0, 110.9, 52.5, 46.1, 32.8, 32.5, 29.8, 28.8, 27.2, 26.9. HRMS (ESI): calcd for C28H28N2Na: 415.21502; found: 415.21538.
1). IR (Neat) νmax: 3408, 3056, 2923, 2853, 1703, 1619, 1583, 1517, 1485, 1455, 1338, 1227, 1095, 1012, 741 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.97 (s, 1H), 7.89 (s, 1H), 7.59–7.57 (d, J = 8 Hz, 2H), 7.37–7.34 (m, 2H), 7.21–7.16 (m, 2H), 7.07–7.05 (m, 2H), 6.98–6.93 (m, 2H), 6.76 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 6.04 (dd, J1 = 5.5 Hz, J2 = 3 Hz, 1H), 4.27 (s, 1H), 4.18 (brs, 1H), 2.57–2.51 (m, 2H), 2.25–2.19 (m, 1H), 2.07–2.06 (m, 1H), 1.72–1.29 (m, 8H). 13C NMR (125 MHz, CDCl3, TMS): δ 142.1, 136.9, 136.7, 135.9, 134.3, 130.4, 129.0, 128.2, 126.7, 126.6, 121.9, 121.5, 120.8, 120.7, 120.2, 120.1, 119.2, 119.0, 111.0, 110.9, 52.5, 46.1, 32.8, 32.5, 29.8, 28.8, 27.2, 26.9. HRMS (ESI): calcd for C28H28N2Na: 415.21502; found: 415.21538.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3289, 3066, 2931, 2857, 1668, 1620, 1582, 1520, 1455, 1304, 1238, 933, 744 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.88 (s, 1H), 7.79 (s, 1H), 7.62 (d, J = 8 Hz, 1H), 7.55 (d, J = 8 Hz, 1H), 7.54–7.21 (m, 2H), 7.19–7.13 (m, 2H), 7.05–6.93 (m, 4H), 6.72–6.71 (m, 1H), 5.94 (dd, J1 = 5.5 Hz, J2 = 3 Hz, 1H), 4.30 (s, 1H), 4.11 (brs, 1H), 3.12 (brs, 1H), 2.49 (brs, 1H), 2.04–1.68 (m, 9H), 1.53–1.43 (m, 2H), 0.88–0.84 (m, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 140.8, 137.8, 137.0, 136.9, 135.4, 135.2, 129.5, 129.1, 128.3, 126.8, 126.5, 125.4, 122.0, 121.8, 120.9, 120.4, 119.2, 118.9, 111.2, 111.1, 52.4, 45.2, 39.8, 39.4, 38.2, 37.3, 35.1, 34.8, 28.4, 21.6. HRMS (ESI): calcd for C31H30N2Na: 453.23067; found: 453.23101.
1). IR (Neat) νmax: 3289, 3066, 2931, 2857, 1668, 1620, 1582, 1520, 1455, 1304, 1238, 933, 744 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.88 (s, 1H), 7.79 (s, 1H), 7.62 (d, J = 8 Hz, 1H), 7.55 (d, J = 8 Hz, 1H), 7.54–7.21 (m, 2H), 7.19–7.13 (m, 2H), 7.05–6.93 (m, 4H), 6.72–6.71 (m, 1H), 5.94 (dd, J1 = 5.5 Hz, J2 = 3 Hz, 1H), 4.30 (s, 1H), 4.11 (brs, 1H), 3.12 (brs, 1H), 2.49 (brs, 1H), 2.04–1.68 (m, 9H), 1.53–1.43 (m, 2H), 0.88–0.84 (m, 1H). 13C NMR (125 MHz, CDCl3, TMS): δ 140.8, 137.8, 137.0, 136.9, 135.4, 135.2, 129.5, 129.1, 128.3, 126.8, 126.5, 125.4, 122.0, 121.8, 120.9, 120.4, 119.2, 118.9, 111.2, 111.1, 52.4, 45.2, 39.8, 39.4, 38.2, 37.3, 35.1, 34.8, 28.4, 21.6. HRMS (ESI): calcd for C31H30N2Na: 453.23067; found: 453.23101.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3090, 2950, 2932, 2857, 1688, 1621, 1583, 1514, 1462, 1380, 1304, 1238, 1108, 1042, 957, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.65 (d, J = 8 Hz, 1H), 7.58 (d, J = 8 Hz, 1H), 7.33–7.20 (m, 6H), 7.08–7.02 (m, 2H), 6.89 (s, 1H), 6.83 (s, 1H), 6.73 (d, J = 5.5 Hz, 1H), 5.95 (t, J = 2.5 Hz, 1H), 4.32 (s, 1H), 4.13 (s, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 3.17 (s, 1H), 2.53 (s, 1H), 2.01–1.58 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 140.6, 137.6, 135.6, 135.2, 129.2, 127.2, 127.0, 125.6, 125.5, 121.5, 120.5, 120.3, 118.8, 118.8, 118.6, 109.1, 108.9, 52.5, 45.2, 39.8, 39.3, 38.2, 37.3, 35.0, 34.4, 32.6, 32.5, 28.4, 28.3. HRMS (ESI): calcd for C33H34N2Na: 481.26197; found: 481.26141.
1). IR (Neat) νmax: 3090, 2950, 2932, 2857, 1688, 1621, 1583, 1514, 1462, 1380, 1304, 1238, 1108, 1042, 957, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.65 (d, J = 8 Hz, 1H), 7.58 (d, J = 8 Hz, 1H), 7.33–7.20 (m, 6H), 7.08–7.02 (m, 2H), 6.89 (s, 1H), 6.83 (s, 1H), 6.73 (d, J = 5.5 Hz, 1H), 5.95 (t, J = 2.5 Hz, 1H), 4.32 (s, 1H), 4.13 (s, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 3.17 (s, 1H), 2.53 (s, 1H), 2.01–1.58 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 140.6, 137.6, 135.6, 135.2, 129.2, 127.2, 127.0, 125.6, 125.5, 121.5, 120.5, 120.3, 118.8, 118.8, 118.6, 109.1, 108.9, 52.5, 45.2, 39.8, 39.3, 38.2, 37.3, 35.0, 34.4, 32.6, 32.5, 28.4, 28.3. HRMS (ESI): calcd for C33H34N2Na: 481.26197; found: 481.26141.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 2981, 2915, 2833, 1671, 1621, 1586, 1542, 1380, 1300, 1238, 1042, 957, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.62–7.47 (m, 6H), 7.38–6.96 (m, 12H), 6.66 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 6.04 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 4.41 (brs, 1H), 4.22 (brs, 1H), 3.61 (s, 3H), 3.57 (s, 3H), 2.98 (brs, 1H), 2.32 (brs, 1H), 1.83–1.50 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 140.2, 137.9, 137.4, 136.2, 135.8, 134.6, 133.9, 133.4, 131.3, 130.5, 129.7, 128.7, 128.2, 127.7, 127.5, 126.6, 125.3, 122.3, 121.4, 120.3, 119.5, 119.1, 119.0, 117.5, 115.2, 108.9, 108.7, 51.0, 45.3, 39.3, 38.9, 37.8, 37.4, 34.9, 33.1, 30.9, 28.1. HRMS (ESI): calcd for C45H42N2Na: 633.32457; found: 633.32486.
1). IR (Neat) νmax: 2981, 2915, 2833, 1671, 1621, 1586, 1542, 1380, 1300, 1238, 1042, 957, 931, 743 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.62–7.47 (m, 6H), 7.38–6.96 (m, 12H), 6.66 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 6.04 (dd, J1 = 5.5 Hz, J2 = 2.5 Hz, 1H), 4.41 (brs, 1H), 4.22 (brs, 1H), 3.61 (s, 3H), 3.57 (s, 3H), 2.98 (brs, 1H), 2.32 (brs, 1H), 1.83–1.50 (m, 12H). 13C NMR (125 MHz, CDCl3, TMS): δ 140.2, 137.9, 137.4, 136.2, 135.8, 134.6, 133.9, 133.4, 131.3, 130.5, 129.7, 128.7, 128.2, 127.7, 127.5, 126.6, 125.3, 122.3, 121.4, 120.3, 119.5, 119.1, 119.0, 117.5, 115.2, 108.9, 108.7, 51.0, 45.3, 39.3, 38.9, 37.8, 37.4, 34.9, 33.1, 30.9, 28.1. HRMS (ESI): calcd for C45H42N2Na: 633.32457; found: 633.32486.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3294, 2857, 2366, 2335, 1647, 1590, 1369, 1120, 1037, 702 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.90 (s, 1H), 7.67–7.51 (m, 3H), 7.37–7.06 (m, 9H), 6.98–6.78 (m, 9H), 6.40 (d, J = 4 Hz, 1H), 6.24 (brs, 1H), 4.51–4.49 (m, 2H). 13C NMR (125 MHz, CDCl3, TMS): δ 148.1, 143.2, 142.6, 140.4, 136.8, 136.5, 135.0, 133.1, 129.8, 129.3, 127.9, 127.4, 126.7, 126.5, 126.2, 125.9, 122.0, 121.5, 120.8, 120.1, 119.8, 119.5, 119.3, 119.0, 118.9, 111.1, 110.9, 57.7, 48.5. HRMS (ESI): calcd for C34H26N2Na: 485.19937; found: 485.19969.
1). IR (Neat) νmax: 3294, 2857, 2366, 2335, 1647, 1590, 1369, 1120, 1037, 702 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.90 (s, 1H), 7.67–7.51 (m, 3H), 7.37–7.06 (m, 9H), 6.98–6.78 (m, 9H), 6.40 (d, J = 4 Hz, 1H), 6.24 (brs, 1H), 4.51–4.49 (m, 2H). 13C NMR (125 MHz, CDCl3, TMS): δ 148.1, 143.2, 142.6, 140.4, 136.8, 136.5, 135.0, 133.1, 129.8, 129.3, 127.9, 127.4, 126.7, 126.5, 126.2, 125.9, 122.0, 121.5, 120.8, 120.1, 119.8, 119.5, 119.3, 119.0, 118.9, 111.1, 110.9, 57.7, 48.5. HRMS (ESI): calcd for C34H26N2Na: 485.19937; found: 485.19969.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (Neat) νmax: 3053, 2927, 1709, 1688, 1613, 1513, 1469, 1427, 1372, 1328, 1242, 1156, 1130, 1013, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.78–7.76 (m, 2H), 7.72–7.30 (m, 9H), 7.20–6.99 (m, 6H), 6.87 (brs, 3H), 6.52 (t, J = 3 Hz, 1H), 6.09 (dd, J1 = 4 Hz, J2 = 2.5 Hz, 1H), 4.66 (brs, 1H), 4.57–4.54 (m, 1H), 3.81 (s, 3H), 3.58 (s, 3H). 13C NMR (125 MHz, CDCl3, TMS): δ 148.8, 143.3, 143.0, 140.8, 137.6, 137.3, 134.9, 132.9, 129.9, 129.3, 129.2, 128.4, 128.0, 127.4, 127.2, 127.2, 126.8, 126.5, 125.8, 125.7, 121.7, 121.1, 120.3, 120.0, 118.9, 118.4, 118.1, 117.6, 109.3, 109.1, 51.8, 48.9, 32.6, 32.2. MS (ESI): calcd for C36H30N2Na: 513.23067; found: 513.23098.
1). IR (Neat) νmax: 3053, 2927, 1709, 1688, 1613, 1513, 1469, 1427, 1372, 1328, 1242, 1156, 1130, 1013, 740 cm−1. 1H NMR (500 MHz, CDCl3, TMS): δ 7.78–7.76 (m, 2H), 7.72–7.30 (m, 9H), 7.20–6.99 (m, 6H), 6.87 (brs, 3H), 6.52 (t, J = 3 Hz, 1H), 6.09 (dd, J1 = 4 Hz, J2 = 2.5 Hz, 1H), 4.66 (brs, 1H), 4.57–4.54 (m, 1H), 3.81 (s, 3H), 3.58 (s, 3H). 13C NMR (125 MHz, CDCl3, TMS): δ 148.8, 143.3, 143.0, 140.8, 137.6, 137.3, 134.9, 132.9, 129.9, 129.3, 129.2, 128.4, 128.0, 127.4, 127.2, 127.2, 126.8, 126.5, 125.8, 125.7, 121.7, 121.1, 120.3, 120.0, 118.9, 118.4, 118.1, 117.6, 109.3, 109.1, 51.8, 48.9, 32.6, 32.2. MS (ESI): calcd for C36H30N2Na: 513.23067; found: 513.23098.Acknowledgements
SCS, PS and PP thank UGC for a research fellowship. Financial assistance from Department of Science and Technology (DST no.: SB/S1/OC-24/2014), Indo-French Centre for Promotion of Advanced Research (IFCPAR/CEFIPRA (Project 4505-1)), and Council of Scientific and Industrial Research (12th FYP project, ORIGIN-CSC-0108), New Delhi are greatly acknowledged. Thanks are due to Dr K. M. Sureshan and Mr Alex Andrews of IISER Trivandrum, for single crystal X-ray analysis. The authors also thank Mrs Saumini Mathew, Mrs S. Viji and Mr Saran P. Raveendran of CSIR-NIIST and Mr B. Adarsh of IISER Trivandrum for recording NMR and mass spectra.Notes and references
- (a) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608–9644 CrossRef CAS PubMed; (b) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489–4497 CrossRef CAS PubMed; (c) R. J. Sundberg, The Chemistry of Indoles, Academic press, New York, 1996, p. 113 Search PubMed.
- M. Berger, J. A. Gray and B. L. Roth, Annu. Rev. Med., 2009, 60, 355–366 CrossRef CAS PubMed.
- (a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873–2920 CrossRef CAS PubMed; (b) K. Krüger (née Alex), A. Tillack and M. Beller, Adv. Synth. Catal., 2008, 350, 2153–2167 CrossRef PubMed; (c) J. Zhou and Y. Tang, J. Am. Chem. Soc., 2002, 124, 9030–9031 CrossRef CAS PubMed; (d) K. B. Jensen, J. Thorhauge, R. G. Hazell and K. A. Jørgensen, Angew. Chem., Int. Ed., 2001, 40, 160–163 CrossRef CAS.
- (a) R. J. Phipps, N. P. Grimster and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 8172–8174 CrossRef CAS PubMed; (b) S. M. A. H. Siddiki, K. Kon and K.-I. Shimizu, Chem.–Eur. J., 2013, 19, 14416–14419 CrossRef CAS PubMed; (c) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449–4465 RSC; (d) G. Yan, C. Kuang, Y. Zhang and J. Wang, Org. Lett., 2010, 12, 1052–1055 CrossRef CAS PubMed; (e) A. Mahadevan, H. Sard, M. Gonzalez and J. C. McKew, Tetrahedron Lett., 2003, 44, 4589–4591 CrossRef CAS; (f) Y. L. Hu, H. Jiang and M. Lu, Green Chem., 2011, 13, 3079–3087 RSC; (g) D. A. Evans, K. A. Scheidt, K. R. Fandrick, H. W. Lam and J. Wu, J. Am. Chem. Soc., 2003, 125, 10780–10781 CrossRef CAS PubMed; (h) G. Blay, I. Fernandez, J. R. Pedro and C. Vila, Org. Lett., 2007, 9, 2601–2604 CrossRef CAS PubMed; (i) K. Gao and J. Wu, Org. Lett., 2008, 10, 2251–2254 CrossRef CAS PubMed.
- (a) G. A. Olah, R. Krishnamurti and G. K. S. Prakash, Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, Oxford, 1991, vol. 3, p. 293 Search PubMed; (b) K. Manabe, N. Aoyama and S. Kobayashi, Adv. Synth. Catal., 2001, 343, 174–176 CrossRef CAS.
- H. D. King, Z. Meng, J. A. Deskus, C. P. Sloan, Q. Gao, B. R. Beno, E. S. Kozlowski, M. A. Lapaglia, G. K. Mattson, T. F. Molski, M. T. Taber, N. J. Lodge, R. J. Mattson and J. E. Macor, J. Med. Chem., 2010, 53, 7564–7572 CrossRef CAS PubMed.
- A. W. Schmidt, K. R. Reddy and H. Knolker, Chem. Rev., 2012, 112, 3193–3228 CrossRef CAS PubMed.
- (a) K. S. Ryan and C. L. Drennan, Chem. Biol., 2009, 16, 351–364 CrossRef CAS PubMed; (b) M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250–2293 CrossRef CAS PubMed; (c) J. S. Oakdale and D. L. Boger, Org. Lett., 2010, 12, 1132–1134 CrossRef CAS PubMed; (d) T. Haahimoto, A. Yasuda, K. Akazawa, S. Takaoka, M. Tori and Y. Asakawa, Tetrahedron Lett., 1994, 35, 2559–2560 CrossRef.
- (a) D. D. Sternbach and C. L. Ensinger, J. Org. Chem., 1990, 55, 2725–2736 CrossRef CAS; (b) K. Sakai and T. Kobori, Tetrahedron Lett., 1981, 22, 115–118 CrossRef CAS; (c) B. Lei and A. G. Fallis, J. Am. Chem. Soc., 1990, 112, 4609–4610 CrossRef; (d) V. Nair, S. Kumar and P. G. Williard, Tetrahedron Lett., 1995, 35, 1605–1608 CrossRef; (e) V. Nair, C. N. Jayan, K. V. Radhakrishnan, G. Anilkumar and N. P. Rath, Tetrahedron, 2001, 57, 5807–5813 CrossRef CAS; (f) J. M. Kuthanapillil, S. Thulasi, R. Rajan, K. S. Krishnan, E. Suresh and K. V. Radhakrishnan, Tetrahedron, 2011, 67, 1272–1280 CrossRef CAS PubMed; (g) K. S. Krishnan, M. M. Bhadbhade, G. V. Bhosekar and K. V. Radhakrishnan, Tetrahedron Lett., 2005, 46, 4785–4788 CrossRef PubMed; (h) K. S. Krishnan, V. S. Sajisha, S. Anas, C. H. Suresh, M. M. Bhadbhade, G. V. Bhosekar and K. V. Radhakrishnan, Tetrahedron, 2006, 62, 5952–5961 CrossRef CAS PubMed.
- (a) A. P. Luna, M. Cesario, M. Bonin and L. Micouin, Org. Lett., 2003, 5, 4771–4774 CrossRef CAS PubMed; (b) S. Crotti, F. Bertolini, F. Macchia and M. Pineschi, Adv. Synth. Catal., 2009, 351, 869–873 CrossRef CAS PubMed; (c) A. Martins, S. Lemouzy and M. Lautens, Org. Lett., 2009, 11, 181–183 CrossRef CAS PubMed; (d) C. Bournaud, F. Chung, A. Luna, M. Pasco, G. Errasti, T. Lecourt and L. Micouin, Synthesis, 2009, 869–887 CAS.
- (a) V. S. Sajisha and K. V. Radhakrishnan, Adv. Synth. Catal., 2006, 348, 924–930 CrossRef CAS PubMed; (b) J. John, V. S. Sajisha, S. Mohanlal and K. V. Radhakrishnan, Chem. Commun., 2006, 3510–3512 RSC; (c) J. John, S. Anas, V. S. Sajisha, S. Viji and K. V. Radhakrishnan, Tetrahedron Lett., 2007, 48, 7225–7227 CrossRef CAS PubMed; (d) J. John, U. Indu, E. Suresh and K. V. Radhakrishnan, J. Am. Chem. Soc., 2009, 131, 5042–5043 CrossRef CAS PubMed; (e) R. Rajan, J. John, S. Thulasi, N. Joseph, K. Radhakrishnan and R. Sawant, Synthesis, 2010, 3649–3656 CAS; (f) N. Joseph, J. John, R. Rajan, S. Thulasi, A. Mohan, E. Suresh and K. V. Radhakrishnan, Tetrahedron, 2011, 67, 4905–4913 CrossRef CAS PubMed; (g) J. John, R. Rajan, S. S. Chand, P. Prakash, N. Joseph, E. Suresh and K. V. Radhakrishnan, Tetrahedron, 2013, 69, 152–159 CrossRef CAS PubMed; (h) P. Prakash, P. Aparna, E. Jijy, P. Santhini, S. Varughese and K. Radhakrishnan, Synlett, 2013, 25, 275–279 CrossRef PubMed; (i) P. Prakash, E. Jijy, P. Preethanuj, P. M. Pihko, S. S. Chand and K. V. Radhakrishnan, Chem.–Eur. J., 2013, 19, 10473–10477 CrossRef CAS PubMed; (j) E. Jijy, P. Prakash, M. Shimi, P. M. Pihko, N. Joseph and K. V. Radhakrishnan, Chem. Commun., 2013, 49, 7349–7351 RSC; (k) E. Jijy, P. Prakash, S. Saranya, E. Suresh and K. V. Radhakrishnan, Synthesis, 2013, 2583–2592 CAS.
- (a) S. S. Chand, E. Jijy, P. Prakash, J. Szymoniak, P. Preethanuj, B. P. Dhanya and K. V. Radhakrishnan, Org. Lett., 2013, 15, 3338–3341 CrossRef CAS PubMed; (b) S. S. Chand, S. Saranya, P. Preethanuj, B. P. Dhanya, E. Jijy, P. Prakash, B. S. Sasidhar, J. Szymoniak, P. V. Santhini and K. V. Radhakrishnan, Org. Biomol. Chem., 2014, 12, 3045–3061 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of all new compounds. CCDC 1034718 and 989506. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra01107h |
| This journal is © The Royal Society of Chemistry 2015 |