Palladium-Schiff-base-silica framework as a robust and recyclable catalyst for Suzuki–Miyaura cross-coupling in aqueous media†
Abstract
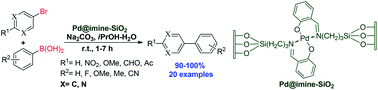
A silica supported palladium catalyst, Pd@imine–SiO2 was prepared by immobilizing Pd(OAc)2 onto silica gel through coordination of imine, generated via Schiff-base condensation between 3-aminopropyltriethoxysilane (APTES) functionalized silica gel and salicylaldehyde. The prepared catalyst was characterized by FT-IR, BET surface area measurements, XRD, SEM-EDX, EDS-mapping and ICP-AES analysis. The imine-based catalyst exhibited excellent activity in the Suzuki–Miyaura cross-coupling reactions of aryl bromides with arylboronic acids in iPrOH/H2O (1 : 1) at room temperature. The reaction proceeds under mild reaction conditions and the catalyst is recyclable, thus offering an environmentally benign alternative to the existing protocols.

 Please wait while we load your content...
Please wait while we load your content...