Open Access Article
Open Access ArticleSynthesis of D–A polymers with a disilanobithiophene donor and a pyridine or pyrazine acceptor and their applications to dye-sensitized solar cells†
Joji
Ohshita
*,
Yohei
Adachi
,
Daiki
Tanaka
,
Makoto
Nakashima
and
Yousuke
Ooyama
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan. E-mail: jo@hiroshima-u.ac.jp; Fax: +81-82-424-5494; Tel: +81-824-424-7743
First published on 10th April 2015
Abstract
New donor–acceptor polymers containing disilanobithiophene (DSBT) as the donor and pyridine or pyrazine as the acceptor with or without a thiophene spacer were prepared. The polymers showed UV-vis absorption maxima at λmax = 488–526 nm, which were red-shifted relative to those of model monomers dithienylpyridine and dithienylpyrazine (λmax = 352–375 nm), indicating the efficient conjugation along the polymer chains. A homo polymer of DSBT was also prepared. The DSBT-containing polymers were attached to TiO2 electrodes by immersing the electrodes in the polymer solutions under irradiation (>400 nm) or in the dark. The modified electrodes were applied to dye-sensitized solar cells and a maximal power conversion efficiency of 0.89% was obtained using the TiO2 electrode thermally modified with a DSBT–pyrazine alternating polymer.
Introduction
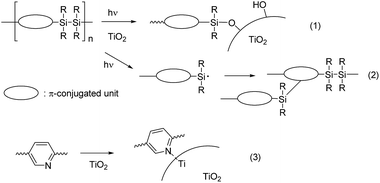
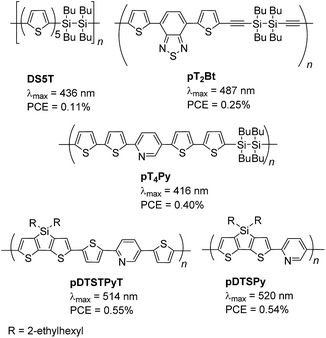
Conjugated donor–acceptor (D–A) compounds and polymers are of current interest because they possess optoelectronic functionalities, including photovoltaic properties.1 Their broad and red-shifted absorption arising from their low band gaps make it possible to utilize the wide sunlight wavelength range, and their polar structures facilitate photo-induced charge separation in this system. Dye-sensitized solar cells (DSSCs) possess TiO2 electrodes modified with sensitizing dyes. The dyes play a crucial role: electron injection from the photoexcited dyes into TiO2 is the key step in the photocurrent generation.2 The dyes usually have a D–A structure and an anchor unit, such as a carboxylic acid or a phosphoric acid, which forms an ester linkage with the TiO2 surface. However, the ester linkages between the sensitizing dyes and the TiO2 surface are generally labile and readily hydrolyzed by moisture, resulting in the detachment of the dyes from the TiO2 surface. Recently, we introduced disilane as a new anchor group that reacts with the TiO2 surface under UV irradiation to form a chemically and thermally stable Si–O–Ti linkage3 (Fig. 1(1)).4 In fact, irradiation of TiO2 electrodes in solutions of poly(disilanyleneoligothienylene)s (DSxT, x = 5 or 6) led to the formation of polymer-attached electrodes (Chart 1) that could be used for DSSCs.4a,b However, DSSCs based on DSxT showed low performance, namely, a maximal power conversion efficiency (PCE) of 0.11%, which is likely due to their narrow absorption band in the visible region.4a More recently, we prepared disilanylene polymers with conjugated D–A systems and used them as sensitizing dyes.4c,d As expected, the introduction of the D–A structure into the polymer backbone improved DSSC performance due to the red-shifted broad absorption band, and resulted in a higher PCE of 0.25% (pT2Bt in Chart 1).4c The introduction of a pyridine (Py) unit into the polymer as the acceptor gave rise to an even higher DSSC performance (PCE = 0.40% for pT4Py),4d likely because the Lewis basic Py unit formed a complex with a Lewis acidic site of the TiO2 surface providing secondary bonding that facilitated the dye attachment (Fig. 1(3)).5 However, in those systems, the photodegradation of the disilanylene polymer by the addition of silyl radicals generated photochemically from the disilane units to the π-conjugated units competed with the formation of the Si–O–Ti linkage to suppress the DSSC performance (Fig. 1(2)).6 Py polymers pDTSTPyT and pDTSPy without disilane bonds were then examined and found to show slightly higher PCEs of 0.55% and 0.54%, respectively.7Recently, we introduced disilanobithiophene (DSBT) as a new donor component of conjugated D–A polymers, and demonstrated its application to bulk hetero-junction polymer solar cells.8 In this paper, we report the synthesis of new D–A polymers with DSBT donor and pyridine or pyrazine (Py or Pz) acceptor. Their optical properties and applications to DSSCs are described. The photochemically generated silyl radicals in DSBT would undergo facial recombination to recover the Si–Si bond, thereby avoiding their addition to the π-conjugated units. The Py and Pz units would coordinate to the Lewis acid center of TiO2, thus making multiple binding of the polymer chain to the surface possible. We investigated also a homo polymer of DSBT to understand the roles of the Py and Pz units.
Experimental†
Synthesis of pDSBT
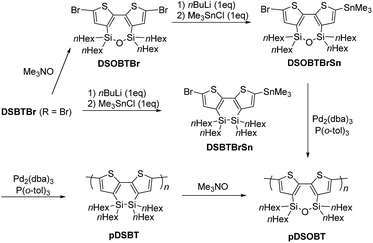
A mixture of 325 mg (0.405 mmol) of DSBTBrSn, 18.5 mg (5.0 mol%) of Pd2(dba)3, 24.7 mg (20.0 mol%) of P(o-tol)3, and 8 mL of toluene was heated at 75 °C for 96 h. As a usual workup process, an aqueous solution of sodium N,N-diethyldithiocarbamate trihydrate was added to the reaction mixture, and the mixture was heated at 80 °C for 2 h. The organic layer was separated and washed with water, 3 vol% acetic acid aqueous solution, and water again in that order. The organic layer was dried over anhydrous magnesium sulfate and the solvent was evaporated. The residue was reprecipitated from toluene/methanol and then toluene/ethanol to give 209 mg (92% yield) of pDSBT as a purple solid. Mp 209–258 °C. 1H NMR (in C6D6): δ = 0.68–1.17 (20H, m, nHex), 1.23–1.45 (24H, m, nHex), 1.51–1.71 (8H, m, nHex), 7.47 (2H, br s, thiophene). 13C NMR (in C6D6): δ = 13.48, 14.23, 16.25 (nHexSiO), 22.99, 23.47 (nHexSiO), 25.46, 31.84, 33.28 (nHexSiO), 33.69, 130.76, 135.94 (2C), 145.09.Synthesis of pDSOBT
A mixture of 222 mg (0.272 mmol) of DSOBTBrSn, 12.5 mg (5.0 mol%) of Pd2(dba)3, 16.5 mg (20.0 mol%) of P(o-tol)3, and 7 mL of toluene was heated at 75 °C for 96 h. After usual workup as above, the residue was reprecipitated from toluene/methanol to give 98.7 mg (63% yield) of pDSOBT as a red solid. Mp 110–158 °C. 1H NMR (in C6D6): δ = 0.86–1.03 (20H, m, nHex), 1.22–1.43 (24H, m, nHex), 1.51–1.68 (8H, m, nHex), 7.43 (2H, br s, thiophene). 13C NMR (in C6D6): δ = 14.29, 16.21, 22.93, 23.46, 31.92, 33.28, 131.08, 137.83, 140.31, 145.39.Alternatively, pDSOBT was prepared by oxidizing pDSBT. A solution of 49.6 mg (0.446 mmol) of trimethylamine N-oxide dihydrate in 50 mL of toluene was dehydrated by azeotropic distillation of 10 mL of toluene. To this was added 81.5 mg of pDSBT at room temperature, and the mixture was heated to reflux overnight. After hydrolysis with water and evaporation of the solvent from the organic layer, the residue was reprecipitated from toluene/methanol to give pDSOBT as a red solid nearly quantitatively. Its 1H NMR spectrum was consistent with that obtained by the polymerization of DSOBTBrSn.
Synthesis of DSBT-containing D–A polymers
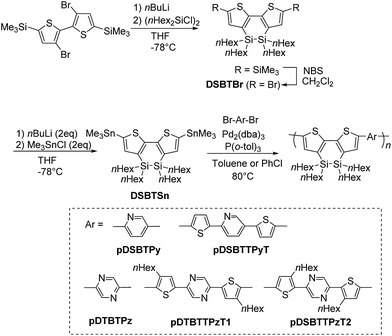
A mixture of 342 mg (0.386 mmol) of DSBTSn, 91.6 mg (0.387 mmol) of 2,5-dibromopyridine, 17.4 mg (5.0 mol%) of Pd2(dba)3, 23.5 mg (20.0 mol%) of P(o-tol)3, and 10 mL of chlorobenzene was heated at 75 °C for 96 h. After usual workup as was done for pDSBT, the residue was reprecipitated from toluene/methanol to give 203 mg (84% yield) of pDSBTPy as a red solid. Mp 64–87 °C. 1H NMR (in CD2Cl2): δ = 0.70–1.08 (20H, m, nHex), 1.16–1.48 (32H, m, nHex), 7.14–7.24 (0.6H, br m, homo-coupled DSBT), 7.42 (0.7H, br s, DSBT), 7.62 (0.7H, br s, DSBT), 7.67–7.76 (0.7H, br m, pyridylene), 7.90 (0.7H, br s, pyridylene), 8.01–8.09 (0.2H, br m, terminal Br-pyridyl), 8.47–8.53 (0.2H, br m, terminal bromopyridyl), 8.85 (0.7H, br s, pyridylene), 8.97 (0.2H, br s, terminal bromopyridyl). 13C NMR (in C6D6): δ = 13.60, 14.21, 16.36 (nHexSiO), 22.99, 23.53 (nHexSiO), 25.53, 31.88, 33.34 (nHexSiO), 33.75. No clear sp2 carbon signals were observed due to the broadening and the low solubility of the polymer.Other DSBT-containing D–A type polymers were obtained in a fashion similar to that above. pDSBTTPyT was purified by reprecipitation from o-dichlorobenzene/methanol. Brown solid. Mp 201–211 °C. 1H NMR (in C6D6): δ = 0.83–1.75 (52H, m, nHex), 6.78–7.64 (8H, br m, aromatic protons), 8.80 (1H, br s, pyridylene). 13C NMR (in C6D6): δ = 13.58, 14.19, 22.98, 25.52, 31.87, 33.70. No clear sp2 carbon signals were observed due to the broadening and the low solubility of the polymer. pDSBTPz was purified by reprecipitation from hot toluene/methanol. Red solid. Mp 77–150 °C. 1H NMR (in CD2Cl2): δ = 0.80–1.10 (20H, m, nHex), 1.18–1.48 (32H, m, nHex), 7.11–7.26 (0.4H, m, homo-coupled DSBT) 7.70 (2H, br s, thiophene), 8.90 (2H, br s, pyrazine). 13C NMR (in C6D6): δ = 13.57, 14.17, 16.33 (nHexSiO), 22.97, 23.53 (nHexSiO), 25.53, 31.88, 33.32 (nHexSiO), 33.72. No clear sp2 carbon signals were observed due to the broadening and the low solubility of the polymer. pDSBTTPzT1 was purified by reprecipitation from hot toluene/methanol. Red solid. Mp 200–226 °C. 1H NMR (in C6D6): δ = 0.86–1.73 (74H, m, nHex), 2.75–2.89 (4H, m, CH2 on thiophene), 7.32 (2H, br s, DSBT thiophene), 7.56 (2H, s, thiophene), 8.64 (2H, s, pyrazine). 13C NMR (in C6D6): δ = 13.52, 14.22, 14.25, 16.39 (nHexSiO), 22.29, 22.30, 23.55 (nHexSiO), 25.52, 29.61, 30.14, 30.82, 31.89, 32.05, 33.36 (nHexSiO), 33.73, 128.67, 132.96, 134.31, 135.23, 135.33, 139.64, 139.91, 141.41, 146.02, 146.32. pDSBTTPzT2 was purified by reprecipitation from hot chlorobenzene/ethyl acetate. Brown solid. Mp 216–248 °C. 1H NMR (in THF-d8): δ = 0.70–1.99 (74H, m, nHex), 2.93–3.16 (4H, br m, CH2 on thiophene), 7.09–7.46 (4H, m, DSBT thiophene), 8.85 (2H, br s, pyrazine). 13C NMR (in C6D6): δ = 13.57, 14.18, 22.98, 25.52, 29.55, 30.59, 31.86, 33.71. No clear sp2 carbon signals were observed due to the broadening and the low solubility of the polymer.
Results and discussion
Polymer synthesis
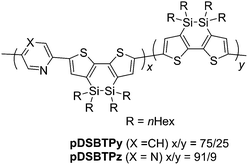
We previously reported the synthesis of a DSBT-benzothiadiazole alternating polymer with butyl groups on the silicon atoms.8 In this study, we employed hexyl-substituted DSBT to increase the solubility of the polymers. Py-containing D–A polymers pDSBTPy and pDSBTTPyT were prepared by the Stille cross-coupling of DSBTSn with 2,5-dibromopyridine and 2,5-bis(5-bromo-2-thienyl)pyridine, respectively, as shown in Scheme 1 and Table 1. In those reactions, DSBTSn that was used as the monomer contained a small amount of the corresponding siloxane (DSOBT) that was formed during the preparation of DSBTBr and could not be separated from either DSBTBr or DSBTSn. The oxidation of DSBT units also occurred during the workup process of the polymers to an extent. The polymers were obtained as dark red solids and could be purified by reprecipitation. The lower yield of pDSBTTPyT than pDSBTPy was due to the formation of insoluble substances that were removed by filtration of the reaction mixture. Polymer molecular weights were determined by GPC (gel permeation chromatography) relative to polystyrene standards. GPC of pDSBTTPyT revealed a bimodal GPC profile with maxima at approximate molecular weights of 7300 and 167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, in contrast to pDSBTPy that showed a monomodal GPC profile. However, the origin of the different GPC profiles of the polymers is not clear yet. A DSBT homo polymer (pDSBT) was also prepared by the Stille coupling of DSBTBrSn as a purple solid (Scheme 2).
000, in contrast to pDSBTPy that showed a monomodal GPC profile. However, the origin of the different GPC profiles of the polymers is not clear yet. A DSBT homo polymer (pDSBT) was also prepared by the Stille coupling of DSBTBrSn as a purple solid (Scheme 2).
| Polymer | Yield/% | Siloxanea/% | GPC | UV absorption | TGc | ||
|---|---|---|---|---|---|---|---|
| M n | M w/Mn | λ max/nm | ε/L g−1 cm−1 | T d 5/°C | |||
| a Siloxane contamination in the backbone, based on 13C NMR spectra. b Could not be determined due to low solubility, but siloxane contamination could be detected by IR spectrum. c In nitrogen at the heating rate of 10 °C min−1. | |||||||
| pDSBT | 92 | 15 | 7200 | 1.95 | 542 | 3.0 × 104 | 425 |
| pDSOBT | 63 | 100 | 8000 | 2.07 | 488 | 2.7 × 104 | 454 |
| pDTBTPy | 77 | 13 | 9500 | 1.86 | 489 | 3.1 × 104 | 419 |
| pDSBTTPyT | 8 | —b | 8700 | 9.50 | 514 | 5.9 × 104 | 445 |
| pDTBTPz | 75 | 22 | 8400 | 2.17 | 522 | 5.4 × 104 | 445 |
| pDSBTTPzT1 | 80 | 3 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.30 | 517 | 6.9 × 104 | 443 |
| pDSBTTPzT2 | 69 | —b | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
4.55 | 526 | 5.9 × 104 | 437 |
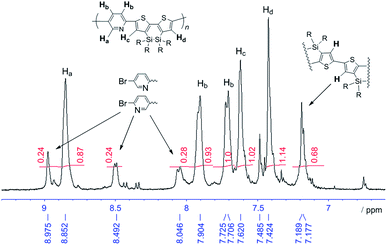
Polymer structures were verified mainly by NMR measurements.† The 1H and 13C NMR spectra of pDSBTTPyT were consistent with the regular alternating structure presented in Scheme 1. In contrast, those of pDSBTPy were rather complex, indicating structural irregularity. Fig. 2 shows the 1H NMR spectrum of pDSBTPy with possible assignment based on the 1H-1H COSY spectrum and comparison of the data with those of the monomers and related compounds, including pDTSPy and pDSBT. The spectrum revealed signals due to homo-coupled DSBT–DSBT units around 7.2 ppm. The incorporation ratio of the homo-coupled units was determined to be approximately x/y = 75/25 (Chart 2) on the basis of the signal integration. Similar homo-coupling was observed in the synthesis of pDTSPy under the same conditions.7 The spectrum also revealed small signals ascribed to terminal bromopyridyl units. The molecular weight calculated based on the integration of the terminal bromopyridyl protons was approximately 7000, in good agreement with that determined by GPC. The contamination ratio of siloxane units in pDSBTPy was determined from the intensities of the hexyl groups in the 13C NMR spectra. The siloxane hexyl carbon signals were identified by comparison with those of pDSOBT that was prepared in two routes, as shown in Scheme 2. The broad Si–O stretching band around 1000 cm−1 in the IR spectra also indicated the existence of the siloxane bonds. For pDSBTTPyT, no homo-coupled units were detected by NMR analysis. Siloxane contamination in pDSBTTPyT was noted from the IR spectrum, but the ratio could not be determined from the 13C NMR spectrum due to the low solubility of the polymer.
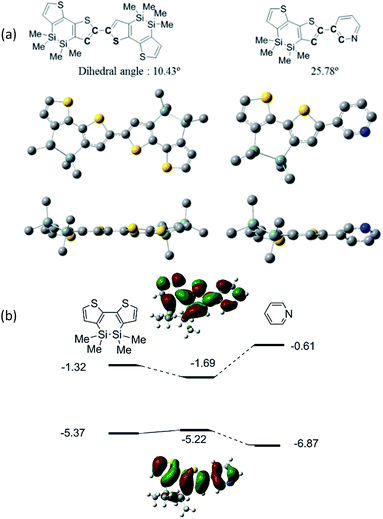
As shown in Table 1 and Fig. 3, the present polymers exhibited broad absorption bands in the UV-vis region, which were red-shifted from those of DSBTBr (λmax = 356 nm) and TPyT (Chart 3), indicating the efficient conjugation between DSBT and Py or TPyT units. However, the absorption bands were blue-shifted from that of pDSBT. The twisting of the DSBT–Py bonds, which arose from the steric repulsion between thiophene and Py C–H bonds, seemed to be responsible for the suppressed conjugation of pDSBTPy and pDSBTTPyT. In fact, computation on models at the B3LYP/6-31G(d) level of theory indicated a larger dihedral angle between the π-conjugated units of DSBT–Py than that of DSBT–DSBT, as shown in Fig. 4a. The rather weak D–A interaction arising from the limited electron-accepting properties of Py would be also one reason for the blue-shifted absorption of the Py polymers. As depicted in Fig. 4b, both the HOMO and LUMO are delocalized over the DSBT–Py π-system, although the HOMO seems to be more DSBT-like judging from that the HOMO energy level is close to that of the DSBT unit. Similarly, an analogous DTS polymer pDTSPy has been reported to exhibit blue-shifted absorption (λmax = 520 nm) relative to that of a DTS homo polymer (λmax = 535 nm).7 The absorption maxima of the present DSBT-polymers were also found at higher energies than those of the corresponding dithienosilole polymers, pDTSPy and pDTSTPyT (λmax = 519 nm). This may be due to the contamination of siloxane units in polymers pDSBTPy and pDSBTTPyT. It has been demonstrated that the conjugation in the silicon-bridged bithiophene is suppressed in the order of DTS9 ≈ DSBT10 > DSOBT,11 depending on the electronic effects of the silicon bridges and the planarity of the bithiophene units (Chart 3). Compared with DTS, DSBT possesses a more twisted bithiophene but exhibits stronger interaction between the silicon σ*-orbital and the bithiophene π*-orbital, thereby lowering LUMO and providing a similar degree of conjugation, as shown in Fig. 4b.8,12 It is also seen that the Si–Si σ-orbital also contributes the HOMO to elevate it. It is also noteworthy that compared with conventional polythiophenes, pDSBT showed expanded conjugation in a manner similar to pDTS (λmax = 545–561 nm), e.g., regio-regular poly(3-hexylthiophene) showed an absorption maximum at 458 nm.13
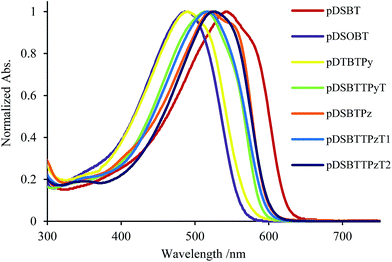 | ||
| Fig. 3 UV-vis spectra of DSBT-containing polymers in dichlrorobenzene or chloroform (for pDSBTTPyT). | ||
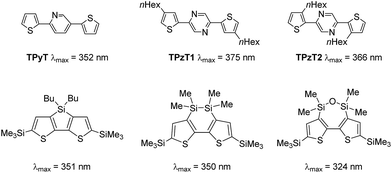 | ||
| Chart 3 UV-vis absorption maxima of dithienylpyridine and dithienylpyrazine, and silicon-bridged bithiophenes. | ||
We prepared Pz-containing polymers pDSBTPz, pDSBTTPzT1, and pDSBTTPzT2, with the expectation that replacement of the Py unit by a sterically less hindered Pz unit would enhance the polymer planarity (Scheme 1). For TPzT units, we introduced solubilizing hexyl groups. As expected, the Pz polymers showed red-shifted absorption maxima relative to those of model monomers TPzT1 and TPzP2 (Chart 3) and Py polymers pDSBTPy and pDSBTTPyT. Higher molecular weight of pDSBTTPzT1, and pDSBTTPzT2 may also be responsible for the red-shifted maxima from that of pDSBTTPyT. In fact, pDSBTTPzT2 with low molecular weight (Mn = 3800 and Mw/Mn = 12.2) that was separated from the polymerization mixture as a solid soluble in ethyl acetate gave rise to a blue-shifted absorption maximum at 514 nm.† However, this was still nearly the same as that of pDSBTTPyT with higher molecular weight Mn = 8700 and Mw/Mn = 9.5, indicative of that Pz-containing polymers possess essentially the more enhanced conjugation. The introduction of alkyl groups on the polymers led to the increased solubility and thus the higher molecular weights of pDSBTTPzT1 and pDSBTTPzT2 than that of pDSBTTPyT. They showed monomodal profile in GPC analysis. The 1H NMR spectrum of pDSBTPz confirmed the existence of the homo-coupled units, similarly to pDSBTPy. However, the incorporation ratio was only x/y = 91/9 (Chart 2), which was much smaller than that of pDSBTPy. It is likely that the formation of sterically less hindered DSBT–Pz bonds proceeded more smoothly than the formation of DSBT–Py bonds. No signals due to the homo-coupled units were observed in the 1H NMR spectra of pDSBTTPzT1 and pDSBTTPzT2.
The thermal stability of the polymers was investigated by thermogravimetric analysis (TGA) in nitrogen and the 5% weight loss temperatures are listed in Table 1. Thermal decomposition occurred at temperatures exceeding 400 °C, indicating the good thermal stability of the present polymers.
Applications to DSSCs
To prepare polymer-attached electrodes, TiO2/FTO electrodes were immersed in chloroform solutions of the present polymers (3 mg mL−1) in argon for 40 min under irradiation (>400 nm) (photochemical conditions) or in the dark (thermal conditions). Homo polymer pDSBT attached to the TiO2 surface efficiently under irradiation, providing a brown electrode. On the other hand, a lightly coloured electrode was obtained in the dark, indicating that the photochemical reactions of pDSBT occurred efficiently on the TiO2 surface, likely by forming Si–O–Ti bonds, although the thermal attachment of the polymer to TiO2 would be also involved to a certain extent. Alternating polymers with Lewis basic Py or Pz units could readily attach to the TiO2 electrodes under both photochemical and thermal conditions. No evident colour difference depending on the conditions was seen. However, the UV-vis spectra of the photochemically modified electrodes always showed blue-shifted absorption bands relative to those of the thermally modified electrodes (Table 2), indicating the photodegradation of chromophores. The Ti–O–Si bond formation would enhance the twisting of bithiophene units. The polymer-attached electrodes were then used for DSSCs with the FTO/polymer-attached TiO2/I−·I3−/Pt structure. All the polymers exhibited sensitizing effects (Fig. 5 and 6) and the cell parameters are summarized in Table 2. The cell with pDSBT showed higher performance when TiO2 was photochemically modified, probably due to the higher degree of polymer attachment. For the cells with Py- or Pz-containing polymers, however, no clear relationship between the performance and the attachment conditions was observed. For some of those cells, it is presumed that sufficient amounts of the polymers could be attached even in the dark. The highest performance was obtained using the electrode thermally modified by pDSBTPz.| Polymer | Methodb | Abs maxc/nm | V oc/mV | J sc/mA cm−2 | FF | PCE (η)/% |
|---|---|---|---|---|---|---|
| a Cell structure: FTO/polymer-attached TiO2/I−·I3−/Pt. b P: under irradiation, T: in the dark. c UV-vis absorption maximum of polymer-attached electrode. | ||||||
| pDSBT | P | 451 | 308 | 2.10 | 0.61 | 0.39 |
| T | 520 | 356 | 0.69 | 0.67 | 0.16 | |
| pDSBTPy | P | 439 | 344 | 1.91 | 0.63 | 0.41 |
| T | 484 | 396 | 1.67 | 0.63 | 0.42 | |
| pDSBTTPyT | P | 468 | 380 | 3.11 | 0.63 | 0.74 |
| T | 475 | 392 | 1.34 | 0.66 | 0.35 | |
| pDSBTPz | P | 468 | 384 | 1.58 | 0.62 | 0.38 |
| T | 496 | 424 | 3.22 | 0.65 | 0.89 | |
| pDSBTTPzT1 | P | 482 | 396 | 2.21 | 0.64 | 0.56 |
| T | 489 | 432 | 2.28 | 0.68 | 0.67 | |
| pDSBTTPzT2 | P | 490 | 384 | 2.70 | 0.59 | 0.61 |
| T | 503 | 420 | 1.58 | 0.66 | 0.44 | |
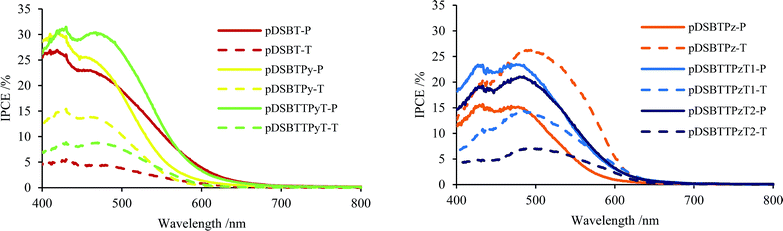 | ||
| Fig. 5 IPCE spectra of TiO2 electrodes modified with DSBT-polymers (P: photochemical conditions, T: thermal conditions). | ||
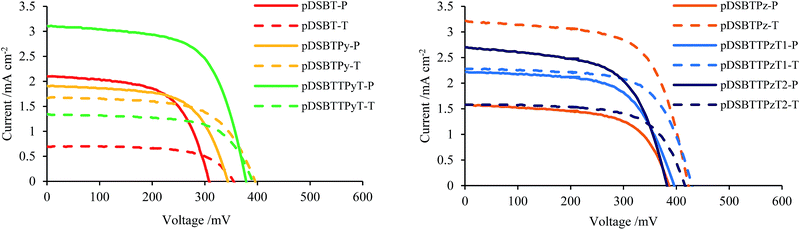 | ||
| Fig. 6 I–V characteristics of DSSCs based on TiO2 electrodes modified with DSBT polymers (P: photochemical conditions, T: thermal conditions). | ||
Quantum chemical simulation for polymer reaction
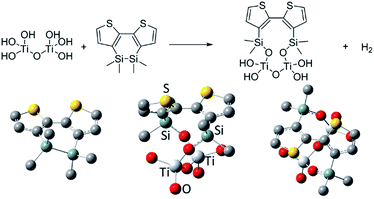
To understand the anchoring reaction of DSBT with the TiO2 surface, we carried out quantum chemical calculations at the B3LYP/6-31+G(dp) level of theory on the Gaussian 09 program, and the results are presented in Fig. 7. The reaction was an exothermic one (−50.1 kcal mol−1), likely due to the formation of thermodynamically stable Si–O bonds. The optimized geometries of DSBT and DSBT-attached TiO2 models indicate that the attachment enhances the twisting of the bithiophene system in which the S–C–C–S dihedral angle is increased from 19.1° to 68.8°. This may be the reason for the blue-shifted absorption of the polymers upon interaction with the TiO2 surface even under thermal conditions.Conclusions
In summary, we prepared new D–A polymers with DSBT as the donor and Py or Pz as the acceptor, and applied them to DSSCs. Although the device performance is still unsatisfactory, this is a new polymer system that is expected to modify inorganic oxide surfaces. Studies to explore polymer functionalities other than DSSC applications are in progress.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (B) (no. 26288094) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.Notes and references
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed.
- Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 18, 2903 CrossRef.
- (a) K. Kakiage, M. Yamamura, E. Fujimura, T. Kyomen, M. Unno and M. Hanaya, Chem. Lett., 2010, 39, 260 CrossRef CAS; (b) M. Unno, K. Kakiage, M. Yamamura, T. Kogure, T. Kyomen and M. Hanaya, Appl. Organomet. Chem., 2010, 24, 247 CrossRef CAS.
- (a) J. Ohshita, J. Matsukawa, M. Hara, A. Kunai, S. Kajiwara, Y. Ooyama, Y. Harima and M. Kakimoto, Chem. Lett., 2008, 37, 316 CrossRef CAS; (b) J. Ohshita, D. Tanaka, J. Matsukawa, T. Mizumo, H. Yoshida, Y. Ooyama and Y. Harima, Chem. Lett., 2011, 40, 87 CrossRef CAS; (c) D. Tanaka, J. Ohshita, Y. Ooyama, T. Mizumo and Y. Harima, J. Organomet. Chem., 2012, 719, 30 CrossRef CAS; (d) D. Tanaka, J. Ohshita, T. Mizumo, Y. Ooyama and Y. Harima, J. Organomet. Chem., 2013, 741–742, 97 CrossRef CAS.
- Y. Ooyama, S. Inoue, T. Nagano, K. Kushimoto, J. Ohshita, I. Imae, K. Komaguchi and Y. Harima, Angew. Chem., Int. Ed., 2011, 50, 7429 CrossRef CAS PubMed.
- (a) J. Ohshita and A. Kunai, Acta Polym., 1998, 49, 379 CrossRef CAS; (b) A. Kunai, T. Ueda, K. Horata, E. Toyoda, I. Nagamoto, J. Ohshita, M. Ishikawa and K. Tanaka, Organometallics, 1996, 15, 2000 CrossRef CAS; (c) J. Ohshita, D. Kanaya and M. Ishimkawa, J. Organomet. Chem., 1994, 468, 55 CrossRef CAS.
- D. Tanaka, J. Ohshita, Y. Ooyama and Y. Morihara, Polym. J., 2013, 45, 1153 CrossRef CAS.
- J. Ohshita, M. Nakashima, D. Tanaka, Y. Morihara, H. Fueno and K. Tanaka, Polym. Chem., 2014, 5, 346 RSC.
- D.-H. Kim, J. Ohshita, K.-H. Lee, Y. Kunugi and A. Kunai, Organometallics, 2006, 25, 1511 CrossRef CAS.
- J. Ohshita, M. Nodono, H. Kai, T. Watanabe, A. Kunai, K. Komaguchi, M. Shiotani, A. Adachi, K. Okita, Y. Harima, K. Yamashita and M. Ishikawa, Organometallics, 1999, 18, 1453 CrossRef CAS.
- Y.-W. Kwak, I.-S. Lee, M.-K. Baek, U. Lee, H.-J. Choi, M. Ishikawa, A. Naka, J. Ohshita, K.-H. Lee and A. Kunai, Organometallics, 2006, 25, 48 CrossRef CAS.
- H. Kai, J. Ohshita, S. Ohara, N. Nakayama, A. Kunai, I.-S. Lee and Y.-W. Kwak, J. Organomet. Chem., 2008, 693, 3490 CrossRef CAS.
- J. Ohshita, K. Kimura, K.-H. Lee, A. Kunai, Y.-W. Kwak, E.-C. Son and Y. Kunugi, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4588 CrossRef CAS.
Footnote |
| † Electronic supplementary information available: Experimental procedures of monomer syntheses, NMR spectra of the polymers, and molecular weight-dependent UV-vis spectra of pDSBTTPzT2. See DOI: 10.1039/c5ra01055a |
| This journal is © The Royal Society of Chemistry 2015 |