Designing breathable superhydrophobic cotton fabrics
Abstract
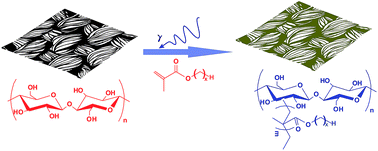
Superhydrophobic cotton fabrics were prepared through radiation induced graft polymerization by applying a series of alkyl methacrylates as the functional monomers. We found that the superhydrophobic cotton fabrics exhibited considerable resistance to air permeability whereas the water vapour transport is almost the same as pristine cotton. The mechanism is that the air permeability is mainly determined by the thickness and pore size of the fabric, while the lower degree of interaction between the grafted cotton fabrics and water molecules along with an enhanced capillary effect are the key factors responsible for the high water vapour transmission rate. The combination of opposing properties – a decreased air permeability and ideal water vapour transmission rate, creates a breathable and comfortable fabric as a dressing material.

 Please wait while we load your content...
Please wait while we load your content...