Influence of ionic liquids as solvents for the chemical synthesis of poly(3-octylthiophene) with FeCl3†
Tae-Joon Parka,
Yong Seok Kimb,
Eunsung Kanc and
Sang Hyun Lee*d
aNano Systems Institute, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 151-744, Korea
bDepartment of Chemical and Biomolecular Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-Gu, Seoul 120-749, Korea
cDepartment of Molecular Bioscience and Bioengineering, University of Hawaii at Manoa, Honolulu, HI 96822, USA
dDepartment of Microbial Engineering, Konkuk University, Hwayang-Dong, Gwangjin-Gu, Seoul 143-701, Korea. E-mail: sanghlee@konkuk.ac.kr; Fax: +82-2-3437-8360; Tel: +82-2-2049-6269
First published on 4th March 2015
Abstract
Ionic liquids (ILs) were used as solvents for the FeCl3-catalyzed oxidative polymerization of 3-octylthiophene (3OT) for the first time. An excellent yield of 99% was obtained by using 1-butyl-3-methylimidazolium hexafluoroantimonate. The effect of the IL structure on the oxidative polymerization of 3OT was analyzed by the linear solvation energy relationship equation.
Among the known conducting polymers, polythiophenes (PTs) are one of the most important conjugated polymers. However, chemical modification of PTs is generally difficult because they are insoluble in most organic solvents, as well as in water. Therefore, the development of highly soluble and easy-to-process PTs is of considerable interest. A number of approaches have utilized incorporation of alkyl, aryl, or sulfonyl groups and carboxyl or sulfonyl groups into thiophenes to achieve dissolution in organic solvents and water, respectively, in order to synthesize various PTs.1,2 Among the substituted thiophenes, alkylthiophenes (ATs) are generally difficult to polymerize because the alkyl chain length of the substituent linked to the thiophenic ring has a significant influence on the electrochemical properties and solubilities of the produced polyalkylthiophenes (PATs) in the reaction media.3 PATs may be prepared either by electrochemical or chemical polymerization methods.4 The first chemical synthesis to prepare poly(2,5-thienylene) using transition metal-catalyzed C–C coupling was reported by Yamamoto et al. in 1980.5 Compared with electrochemical polymerization, chemical polymerization offers several advantages, including a greater selection of monomers, the ability to synthesize perfectly regioregular substituted PATs using proper catalysts, and higher productivity.4,6–9 Oxidative polymerization using inexpensive ferric(III) chloride (FeCl3) at room temperature has been reported for the synthesis of PATs for large-scale production.4,10 However, the selection of solvents as reaction media is still under consideration, because the reaction media can influence the solubility of the monomer, production yield of PATs, and molecular weight of PATs. Chloroform is commonly used to synthesize PATs; however, this solvent has associated environmental and operational issues because of its high toxicity and volatility.4,10
Recently, ionic liquids (ILs) have been exploited as green solvents in the synthesis of conducting polymers via electrochemical polymerization and chemical polymerization.11–15 ILs are organic salts that melt below 100 °C. Their nonvolatile character and thermal stability make them attractive alternatives to volatile organic solvents. In chemical processes, ILs exhibit excellent physical characteristics, including the ability to dissolve polar and nonpolar organic, inorganic, and polymeric compounds. Green polymerization systems employing ILs do not utilize any toxic solvents and the used ILs can be easily recovered after the reaction. Therefore, environmentally friendly, low-cost routes for the synthesis of PATs may be developed by using ILs. ILs have been used as reaction media for the FeCl3-catalyzed reactions. Ji et al. reported the FeCl3-catalyzed electrophilic substitution reactions of indoles with various aldehydes in ILs.16 Pringle et al. showed the FeCl3-catalyzed synthesis of PT nanoparticles in 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([EmIm][Tf2N]).13 Shang et al. reported the synthesis of poly(3-methyl thiophene) nanospheres in magnetic ionic liquid containing [FeCl4−].11 In this study, various ILs are used as reaction media for the chemical synthesis of poly(3-octylthiophene) (P3OT) using FeCl3. First, ILs containing the same cation, 1-butyl-3-methylimidazolium ([BmIm]), were used to investigate the effect of the anion structure on the oxidative polymerization of 3OT. Five counter-anions were employed, namely, [Tf2N], hexafluoroantimonate ([SbF6]), hexafluorophosphate ([PF6]), tetrafluoroborate ([BF4]), and trifluoromethanesulfonate ([OTf]). The effect of the cation structure was studied by using 1-methyl-3-octylimidazolium trifluoromethanesulfonate ([OmIm][OTf]) (Fig. S1, ESI†). Additionally, multiparameter linear regression using the solvatochromic parameters of the ILs was also carried out to elucidate the effect of the solvent parameters of the ILs on the FeCl3-catalyzed oxidative polymerization of 3OT.
Green polymerization in various ILs and conventional polymerization in chloroform were performed at 25 °C for 48 h using 0.42 mmol 3OT as a monomer and 1.68 mmol FeCl3 as a catalyst (Scheme 1). FeCl3-catalyzed oxidative polymerization was successfully achieved in all ILs. The molecular weights, polydispersity indices (PDI), and yields of the P3OTs are listed in Table 1. The yield of conventional polymerization in chloroform, as a control experiment, was 87%. The weight-average molecular weight (Mw) and polydispersity index (PDI) of P3OT produced in chloroform were 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 672 g mol−1 and 12.9, respectively. The highest yield of P3OT achieved to date via FeCl3-catalyzed chemical oxidative polymerization of 3OT was obtained herein with the use of [BmIm][SbF6] (Table S1, ESI†).17–21 A remarkable 99% yield of P3OT with a Mw of 42
672 g mol−1 and 12.9, respectively. The highest yield of P3OT achieved to date via FeCl3-catalyzed chemical oxidative polymerization of 3OT was obtained herein with the use of [BmIm][SbF6] (Table S1, ESI†).17–21 A remarkable 99% yield of P3OT with a Mw of 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 584 g mol−1 and PDI of 15.9 was obtained using [BmIm][SbF6]. The oxidative polymerization of 3OT is initiated by the reaction between 3OT monomer and the Fe3+ ions and then the propagation reactions between the cationic 3OT monomeric radicals are repeated. The high yield of P3OT in [BmIm][SbF6] may be due to the strong acidic condition of SbF6 anion which can help producing radicals in thiophene rings.22 Hence, the proposed alternative synthetic approach for the synthesis of PATs using [BmIm][SbF6] instead of chloroform may have remarkable advantages such as higher product yield, greener reaction medium, easier work-up, and more facile reusability of the reaction medium in large-scale production for the development of environmentally friendly and energy efficient industrial processes.23
584 g mol−1 and PDI of 15.9 was obtained using [BmIm][SbF6]. The oxidative polymerization of 3OT is initiated by the reaction between 3OT monomer and the Fe3+ ions and then the propagation reactions between the cationic 3OT monomeric radicals are repeated. The high yield of P3OT in [BmIm][SbF6] may be due to the strong acidic condition of SbF6 anion which can help producing radicals in thiophene rings.22 Hence, the proposed alternative synthetic approach for the synthesis of PATs using [BmIm][SbF6] instead of chloroform may have remarkable advantages such as higher product yield, greener reaction medium, easier work-up, and more facile reusability of the reaction medium in large-scale production for the development of environmentally friendly and energy efficient industrial processes.23
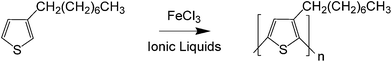 | ||
| Scheme 1 Reaction scheme for the synthesis of P3OT via FeCl3-catalyzed oxidative polymerization in ILs. | ||
| Solvent | Mw (g mol−1) | PDI (Mw/Mn) | Yield (%) |
|---|---|---|---|
| a Synthesis of P3OT was carried out using 83 mg OT, 273 mg FeCl3, and 5 g solvent. Polymerization reactions were performed at 25 °C for 48 h. | |||
| [BmIm][SbF6] | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 584 584 |
15.9 | 99 |
| [BmIm][Tf2N] | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 088 |
10.6 | 51 |
| [BmIm][PF6] | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 921 921 |
19.2 | 45 |
| [BmIm][BF4] | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 444 444 |
17.7 | 44 |
| [BmIm][OTf] | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 109 109 |
15.5 | 38 |
| [OmIm][OTf] | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842 842 |
43.2 | 39 |
| CHCl3 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 672 672 |
12.9 | 87 |
As shown in Table 1, the Mw, PDI, and yield of P3OT were strongly influenced by the structure of the ILs. The highest Mw of P3OT was obtained with [BmIm][BF4] and it was about 1.5 times higher than the Mw of P3OT synthesized in chloroform, whereas the lowest Mw was obtained with [BmIm][Tf2N]. The higher Mw may be derived from the higher solubility of 3OT and P3OT in ILs. The lowest PDI was obtained with [BmIm][Tf2N], whereas the highest PDI was obtained with [OmIm][OTf]. The PDI was highly influenced by the cation structure of the ILs because the PDI of P3OT synthesized in [OmIm][OTf] was much higher than that synthesized in [BmIm][OTf]. It may be caused by the different solubility of P3OT, because the hydrophobicity of [OmIm][OTf] is much higher than that of [BmIm][OTf] while other physicochemical properties of two ILs are very similar. The yield of P3OT synthesized in ILs containing the [BmIm] cation increased in the following order: [BmIm][OTf] < [BmIm][BF4] < [BmIm][PF6] < [BmIm][Tf2N] < [BmIm][SbF6]. Changing the cation structure of the ILs did not enhance the yield of P3OT.
In order to elucidate the effect of the physicochemical properties of the ILs on the oxidative polymerization of 3OT, the correlation between the hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), hydrophilicity (water solubility, log
P), hydrophilicity (water solubility, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sw), dipolarity/polarizability (π*), hydrogen bond acidity (α), and hydrogen bond basicity (β) of the ILs and the Mw, PDI, and yield of P3OT was evaluated. Table 2 lists various parameters of the ILs. The logarithm of the Mw of P3OT was well correlated with the π* value, with a high coefficient of determination (r2) value of 0.788 (n = 6). The Mw of P3OT increased with increasing dipolarity/polarizability of the ILs. On the other hand, the logarithm of the yield of P3OT was well correlated with the β value, with an r2 value of 0.649 (n = 6). The yield of P3OT increased with decreasing hydrogen bond basicity of the ILs. Although the mechanism of FeCl3-catalyzed oxidative polymerization of 3-alkylthiophenes (3AT) is still unclear, Niemi et al. proposed a radical mechanism and reported that FeCl3 must be solid to be active as a polymerization oxidant for 3AT.4 They also reported that polymerization of 3AT did not occur when FeCl3 was completely dissolved in organic solvents. However, these results could be more clearly understood by considering the hydrogen bond basicity of the solvents. The β values of chloroform (0.10), hexane (0.00), and carbon tetrachloride (0.00), in which polymerization can be achieved, are much lower than those of formic acid (0.38), diethylether (0.47), and acetone (0.43), in which polymerization is unsuccessful (Table S2, ESI†).24,25 When the logarithm of the yield of P3OT was correlated with the β values of the ILs and that of chloroform (n = 7), a higher r2 value (0.743) could be obtained than the r2 value (0.649) calculated using the ILs only. This indicates that the hydrogen bond basicity of the solvents is a very important factor for the successful performance of FeCl3-catalyzed oxidative polymerization.
Sw), dipolarity/polarizability (π*), hydrogen bond acidity (α), and hydrogen bond basicity (β) of the ILs and the Mw, PDI, and yield of P3OT was evaluated. Table 2 lists various parameters of the ILs. The logarithm of the Mw of P3OT was well correlated with the π* value, with a high coefficient of determination (r2) value of 0.788 (n = 6). The Mw of P3OT increased with increasing dipolarity/polarizability of the ILs. On the other hand, the logarithm of the yield of P3OT was well correlated with the β value, with an r2 value of 0.649 (n = 6). The yield of P3OT increased with decreasing hydrogen bond basicity of the ILs. Although the mechanism of FeCl3-catalyzed oxidative polymerization of 3-alkylthiophenes (3AT) is still unclear, Niemi et al. proposed a radical mechanism and reported that FeCl3 must be solid to be active as a polymerization oxidant for 3AT.4 They also reported that polymerization of 3AT did not occur when FeCl3 was completely dissolved in organic solvents. However, these results could be more clearly understood by considering the hydrogen bond basicity of the solvents. The β values of chloroform (0.10), hexane (0.00), and carbon tetrachloride (0.00), in which polymerization can be achieved, are much lower than those of formic acid (0.38), diethylether (0.47), and acetone (0.43), in which polymerization is unsuccessful (Table S2, ESI†).24,25 When the logarithm of the yield of P3OT was correlated with the β values of the ILs and that of chloroform (n = 7), a higher r2 value (0.743) could be obtained than the r2 value (0.649) calculated using the ILs only. This indicates that the hydrogen bond basicity of the solvents is a very important factor for the successful performance of FeCl3-catalyzed oxidative polymerization.
| Solvent | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sw Sw |
π* | α | β |
|---|---|---|---|---|---|
| [BmIm][SbF6] | −2.66 | 0.108 | 1.038 | 0.630 | 0.136 |
| [BmIm][Tf2N] | −0.55 | 0.061 | 0.950 | 0.635 | 0.293 |
| [BmIm][PF6] | −2.06 | 0.220 | 0.982 | 0.664 | 0.235 |
| [BmIm][BF4] | −2.71 | 1.121 | 1.036 | 0.626 | 0.450 |
| [BmIm][OTf] | −1.63 | 1.183 | 1.000 | 0.613 | 0.537 |
| [OmIm][OTf] | 0.17 | 1.183 | 0.974 | 0.605 | 0.595 |
| CHCl3 | 1.97 | — | 0.28 | 0.00 | 0.10 |
It is generally difficult to elucidate the effect of a solvent on various physicochemical systems using only one solvent parameter. Therefore, Kamlet et al. developed a linear solvation energy relationship (LSER) using four independent parameters: π*, α, β, and the solubility parameter; the LSER equation has been successfully applied to many equilibrium and kinetic phenomena, including solubilities, partition coefficients, toxicities, and catalytic reactions. The general form of the LSER for the solvent effect is as follows:26
| ΔG = ΔG0 + d2δ1 + s2π*1 + a2a1 + b2β1 + h2(δH)12 | (1) |
| log(yield) = 5.13(±0.87) + 0.63(±0.39)π* − 5.77(±0.98)α − 1.15(±0.11)β | (2) |
 | ||
| Fig. 1 (a) Calculated values of log(yield) against experimental values of log(yield) and (b) linear plot of log(yield) against β values of ILs. | ||
The UV-vis spectra of P3OT synthesized using [BmIm][SbF6] as a solvent were acquired in CHCl3 solutions (Fig. S2, ESI†). In CHCl3 solutions (10 mg mL−1), the most intense maximum peak was observed at 420 nm. Fig. 2 shows the photoluminescence (PL) spectra of monomeric 3OT and P3OT synthesized using various ILs. The intensity of the peak at 560 nm for P3OT synthesized using various ILs was significantly enhanced in comparison with that of monomeric 3OT. This result indicates that P3OT was successfully polymerized by FeCl3 in the ILs and could be useful as a light-emitting polymer in a wide variety of applications such as organic light emitting diodes (OLEDs), optical sensors, and luminescent devices.
Conclusions
An environmentally friendly and energy efficient protocol for the synthesis of P3OT using ILs instead of chloroform was presented. An excellent yield of 99% was obtained by using [BmIm][SbF6]. The molecular weight and yield of P3OT were strongly influenced by the dipolarity/polarizability and hydrogen bond basicity of the ILs, respectively. The yield of P3OT in the ILs was well predicted by multiparameter linear regression using three solvatochromic parameters. FeCl3-catalyzed oxidative polymerization using ILs could have remarkable advantages such as higher product yield, greener reaction conditions, and easier solvent reusability than conventional systems. P3OTs synthesized by using ILs may have potential applications in the field of OLEDs, optical sensors, and luminescent devices.Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (2012M3A9B2030170) and by the Industrial Technology Innovation Program (10050991) funded by the Ministry of Trade, Industry & Energy (MI, Korea). This project was supported by the Korea Ministry of Environment under the “Converging Technology Project (2012-000620001).” This work was carried out with the support of the Rural Development Administration under the “Cooperative Research Program for Agriculture Science & Technology Development (010205022014).” Support was also provided by a Korea CCS R&D Center (KCRC) grant (2013M1A8A1038187) and partial support was provided by an Energy Efficiency & Resources of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korean Government Ministry of Trade, Industry, and Energy (20133030000300).References
- R. D. McCullough, Adv. Mater., 1998, 10, 93 CrossRef CAS.
- I. F. Perepichka, D. F. Perepichka, H. Meng and F. Wudl, Adv. Mater., 2005, 17, 2281 CrossRef CAS.
- A. S. Ribeiro, W. A. Gazotti, V. C. Nogueira, D. A. Machado, P. F. dos Santos Filho and M.-A. De Paoli, J. Chil. Chem. Soc., 2004, 49, 197 CAS.
- V. M. Niemi, P. Knuuttila and J. E. Österholm, Polymer, 1992, 33, 1559 CrossRef CAS.
- T. Yamamoto, K. Sanechika and A. Yamamoto, J. Polym. Sci., Polym. Lett. Ed., 1980, 18, 9 CrossRef CAS.
- R. Sugimoto, S. Takeda, H. B. Gu and K. Yoshino, Chem. Express, 1986, 1, 635 CAS.
- K. Yoshino, S. Hayashi and R. Sugimoto, Jpn. J. Appl. Phys., 1984, 23, 899 CrossRef CAS.
- M. R. Andersson, D. Selse, M. Berggren, H. Järvinen, T. Hijertberg, O. Inganäs, O. Wennerström and J.-E. Österholm, Macromolecules, 1994, 27, 6503 CrossRef CAS.
- F. Andreani, E. Salatelli and M. Lanzi, Polymer, 1996, 37, 661 CrossRef CAS.
- T. Cai, Y. Zhou, E. Wang, S. Hellstrom, F. Zhang, S. Xu, O. Inganas and M. R. Andersson, Sol. Energy Mater. Sol. Cells, 2010, 94, 1275 CrossRef CAS PubMed.
- S. Shang, L. Li, X. Yang and L. Zheng, J. Colloid Interface Sci., 2009, 333, 415 CrossRef CAS PubMed.
- Y. Pang, X. Li, H. Ding, G. Shi and L. Jin, Electrochim. Acta, 2007, 52, 6172 CrossRef CAS PubMed.
- J. M. Pringle, O. Ngamna, J. Chen, G. G. Wallace, M. Forsyth and D. R. MacFarlane, Synth. Met., 2006, 156, 979 CrossRef CAS PubMed.
- K. Sekiguchi, M. Atobe and T. Fuchigami, J. Electroanal. Chem., 2003, 557, 1 CrossRef CAS.
- J. M. Pringle, M. Forsyth, D. R. MacFarlane, K. Wagner, S. B. Hall and D. L. Officer, Polymer, 2005, 46, 2047 CrossRef CAS PubMed.
- S. J. Ji, M. F. Zhou, D. G. Gu, Z. Q. Jiang and T. P. Loh, Eur. J. Org. Chem., 2004, 1584 CrossRef CAS.
- T. Yamamoto, A. Morita, Y. Miyazaki, T. Maruyama, H. Wakayama, Z. H. Zhou, Y. Nakamura, T. Kanbara, S. Sasaki and K. Kubota, Macromolecules, 1992, 25, 1214 CrossRef CAS.
- J.-E. Osterholm, J. Laakso, P. Nyholm, H. Isotalo, H. Stubb, O. Inganäs and W. R. Salaneck, Synth. Met., 1989, 28, C435 CrossRef.
- M. Leclerc, F. M. Diaz and G. Wegner, Makromol. Chem., 1989, 190, 3105 CrossRef CAS.
- M. Pomerantz, J. J. Tseng, H. Zhu, S. J. Sproull, J. R. Reynolds, R. Uitz, H. J. Arnott and H. I. Haider, Synth. Met., 1991, 41–43, 825 CrossRef.
- H. Jarvinen, L. Lahtinen, J. Nasman, O. Hormi and A.-L. Tammi, Synth. Met., 1995, 69, 299 CrossRef.
- Y. S. Kim, S. M. Lee, P. Govindaiah, S. J. Lee, S. H. Lee, J. H. Kim and I. W. Cheong, Synth. Met., 2013, 175, 56 CrossRef CAS PubMed.
- T. Ogoshi, T. Onodera, T. A. Yamagishi and Y. Nakamoto, Macromolecules, 2008, 41, 8533 CrossRef CAS.
- http://www.stenutz.eu/chem/solv26.php.
- K. Hofmann, K. Schreiter, A. Seifert, T. Ruffer, H. Lang and S. Spange, New J. Chem., 2008, 32, 2180 RSC.
- Y. H. Kim, Y. K. Choi, J. Park, S. Lee, Y. H. Yang, H. J. Kim, T. J. Park, Y. H. Kim and S. H. Lee, Bioresour. Technol., 2012, 109, 312 CrossRef CAS PubMed.
- T. P. Wells, J. P. Hallett, C. K. Williams and T. Welton, J. Org. Chem., 2008, 73, 5585 CrossRef CAS PubMed.
- C. Chiappe, M. Malvaldi and C. S. Pomelli, Green Chem., 2010, 12, 1330 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01003a |
| This journal is © The Royal Society of Chemistry 2015 |

