Sequential one-pot approach for the synthesis of functionalized phthalans via Heck-reduction–cyclization (HRC) reactions†
Jonnada Krishna,
Pedireddi Niharika and
Gedu Satyanarayana*
Department of Chemistry, Indian Institute of Technology (IIT), Hyderabad, Ordnance Factory Estate Campus, Yeddumailaram – 502 205, Medak District, Telangana, India. E-mail: gvsatya@iith.ac.in; Fax: +91 40 2301 6032; Tel: +91 40 2301 6054
First published on 26th February 2015
Abstract
An efficient and practical method is described for the direct synthesis of 1,3-dihydroisobenzofurans, an important structural motif present in biologically active natural or synthetic compounds. The reaction was performed in an almost one-pot fashion via controlled [Pd]-catalyzed intermolecular Mizoroki–Heck coupling between 2-bromobenzaldehydes and allylic alcohols followed by reduction and treatment of crude diol with a Lewis acid to give 1,3-dihydroisobenzofurans. Significantly, the method enabled the synthesis of 1,3-dihydroisobenzofurans with simple to dense functionalities on the aromatic rings.
Introduction
Development of new synthetic methods based on one-pot synthesis or sequential one-pot synthesis are considered as important methods for the synthesis of organic compounds.1 Particularly, the sequential one-pot processes involved in multiple reactions are promoted by a metal-catalyst in a sequential fashion.2 Moreover, such transformations could also be feasible via the sequential addition of reagents to accomplish a set of reactions.3 In addition, it would also be possible to develop such one-pot methods using a metal-catalyst to complete the initial reaction followed by the treatment of the resultant intermediate product with a reagent or vice versa.4 These types of processes can be classified into pseudo-domino, cascade5 and tandem-processes. Usually, the one-pot methods have certain advantages over conventional methods. For example, they reduce waste formation, save time, energy, resources and increase the efficacy.6 In general, the overall yields of such processes are found to be higher than those from the corresponding step-wise methods. Thus, the sequential one-pot processes that construct multiple bonds are of immense importance, particularly for the synthesis of cyclic structures because many cyclic systems constitute core or part-structures of biologically active natural or synthetic compounds.Recently, transition-metal mediated one-pot processes have gained considerable attention due to their procedural advantages.7,8 Among them, the domino Heck reactions under [Pd]-catalysis are well known,9–11 although the reports on Heck coupling followed by reduction are limited.12 In continuation of our interest in the development of synthetic methods by [Pd]-catalysis,13 we have observed the selective formation of β-aryl allylic alcohols 3 in a highly regio- and stereo-selective manner.13b Surprisingly, this is not the expected product 3 under conventional Jeffery-Heck conditions. After a careful study of the literature, we realized that the usual Heck reaction followed by double bond isomerization to give the carbonyl compounds was observed for those substrates having no ortho-substituents on the aromatic ring of the allylic alcohols.14 Therefore, it was thought that the bromo substituent at the ortho-position on the aromatic moiety of the allylic alcohol plays a major role to confine the rotation around C–C bond of the PdCH–CH(OH)Ar intermediate (Scheme 1).
Amongst the β-aryl allylic alcohols 3, those with aldehyde functionality on the aromatic ring appear to be a potential synthetic precursor for the synthesis of oxygen containing heterocyclic compounds (i.e., R3 = CHO and X = Br). Therefore, herein, we report a short and efficient synthesis of interesting cyclic ethers 1,3-dihydroisobenzofurans 6 by employing reduction and acid mediated intramolecular cyclization protocol on β-aryl allylic alcohols 3.
Oxygen containing heterocyclic compounds are widely assayed for their substantial therapeutic applications such as tetrahydroisobenzofuran motifs.15,16 They are pervasive structural elements in biologically relevant small molecules (Fig. 1). For example, 3-deoxyisochracinic acid 8 that was isolated from Cladosporium species shows antibacterial activity by inhibiting the growth of Bacillus subtilis.15a The cyclic ether pestacin 9 was obtained from microorganism Pestalotiopsis microspora, which exhibits antifungal, antimycotic and antioxidant activities.15b FR 198248 10 was isolated from Aspergillus flavipes, which shows antibacterial activity and inhibitory activity against Staphylococcus aureus peptide deformylase and also exhibits anti-influenza activity.15c–e 1,4-Dimethoxy-3-(3R-hydroxy-3R-methyl-1-tetralone)-1(3H)-isobenzofuran 11 was isolated from the broth of marine Streptomyces species M268 and identified to be cytotoxic against human cancer cell lines HL-60, A549 and BEL-7402.15f 7-Bromo-1-(2,3-dibromo-4,5-dihydroxyphenyl)-5,6-dihydroxy-1,3-dihydroisobenzofuran 12 was isolated from a brown alga Leathesia nana, and it shows potential action on malignant tumors and cardiovascular diseases.15g The (S)-(+)-enantiomer 13, known as escitalopram, seems to be more potent than the other (S)-(−)-enantiomer.15h–l
We thought that the process can be made more efficient by developing a sequential one-pot method for the direct synthesis of diol 5 starting from aryl allylic alcohols 2 and 2-bromobenzaldehydes 1. This can be achieved by [Pd]-catalysed coupling for the formation of β-aryl allylic alcohols 3 and in situ reduction of the aldehyde functionality. Thus, the [Pd]-catalyzed coupling of 2-bromobenzaldehyde 1c with ortho-bromo aryl allylic alcohol 1a followed by the reduction of 3ca with NaBH4 gave the desired diol 5ca in a very good yield (Scheme 2). The idea behind this hypothesis is to minimize the number of steps and waste and to improve the overall yield of the reaction over the step-wise approach. However, the diol 5ca was not characterized due to its insolubility in CDCl3, and hence it proceeded to the next reaction.
With the required diol 5ca in hand, the acid promoted cyclization was subsequently explored under different sets of conditions, and the results are summarized in Table 1. Thus, the reaction carried out with a Lewis acid (BF3·Et2O) at 0 °C, as well as at −10 °C, leads to the decomposition of the starting material (Table 1, entries 1 and 2). Therefore, the reaction at a further low temperature (−20 °C), furnished the product 6ca in poor yield (30%, Table 1, entry 3). Interestingly, a further drop of temperature (−40 °C) gave the product 6ca in excellent yield (95%, Table 1, entry 4). However, exploring the reaction with different acids such as protic acid (p-TSA) or Lewis acid (AlCl3) furnished the product 6ca in moderate to very good yields (Table 1, entries 5–7), whereas the reaction with H2SO4 gave the product in poor yield (20%, Table 1, entries 8).
| Entrya | Acid (equiv) | Solvent (5 mL) | Temp (°C) | Time min | Yieldb of 6ca (%) |
|---|---|---|---|---|---|
| a Reaction conditions: all the reactions carried out with diol 5ca (0.10 mmol) in DCM.b Isolated yields of chromatographically pure products. | |||||
| 1 | BF3·Et2O (2.0) | DCM | 0 | 15 | — |
| 2 | BF3·Et2O (4.0) | DCM | −10 | 15 | — |
| 3 | BF3·Et2O (5.0) | DCM | −20 | 15 | 30 |
| 4 | BF3·Et2O (5.0) | DCM | −40 | 120 | 95 |
| 5 | p-TSA (3.0) | DCM | −40 | 60 | 50 |
| 6 | AlCl3 (1.2) | DCM | −40 | 10 | 70 |
| 7 | AlCl3 (1.2) | DCE | −40 | 10 | 80 |
| 8 | H2SO4 (3.0) | DCM | −40 | 30 | 20 |
Having established the reaction conditions for the synthesis of 1,3-dihydroisobenzofuran 6, we thought that the method can still be made more efficient by performing cyclization directly on crude diol 5ca without the column purification. Interestingly, the reaction was found to be smooth on the crude diol 5ca (i.e., the crude diol, which was obtained after work-up followed by concentration under reduced pressure) and furnished the product in the overall yield of 48% (Scheme 3). The structure of the cyclic ether 6ca was confirmed from the spectroscopic data. 1H-NMR data unambiguously confirmed the geometry of the double bond as trans by calculating the coupling constant (J = 15.5 to 15.6 Hz, see Experimental section and ESI†). Therefore, the other possibility for the formation of seven membered cyclic ether 7ca was ruled out because it must contain a cis double bond. In addition, the formation of five membered cyclic ether 6ca is geometrically favoured over the seven membered one.
Now, with the optimized reaction conditions in hand, to check the scope and limitations of the method, we investigated this sequential one-pot method on various 2-bromobenzaldehydes 1a–1g in conjunction with ortho-bromo aryl allylic alcohols 2a–2h. Quite interestingly, the method was amenable on various systems possessing dense functionalities on both the aromatic rings and furnished the products 6aa–6gg in moderate yields (41–55%), as summarized in Fig. 2. It is worth mentioning that although the yields of the cyclic ether products 6 are moderate, they actually represent the overall yield of three individual reactions. Therefore, each step contributes to at least 75% yield, and thus the method still stands efficient.
After the successful synthesis of 1,3-dihydroisobenzofurans, we planned to increase the scope of this protocol by employing the allylic alcohols possessing a methyl/methoxy group in the ortho position. During the sequential one-pot approach, we observed the formation of the regular Jeffery-Heck product along with the Mizoroki–Heck product.13b This (Jeffery-Heck product) interfered in the further steps and hindered the isolation of clean products. Thus, we proceeded in a step-wise approach and achieved the targeted 1,3-dihydroisobenzofurans 6ai and 6aj in a moderate overall yield (47% and 52%). It is worth mentioning that in these cases, we were also able to characterise diol 5 (Scheme 4).
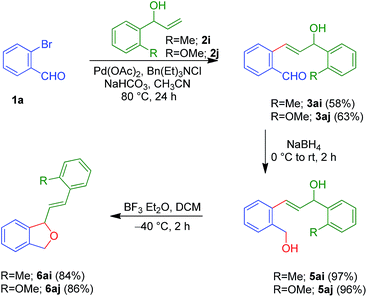 | ||
| Scheme 4 Step-wise approach for the synthesis of 1,3-dihydroisobenzofurans 6ai and 6aj from 2-bromobenzaldehyde 1a and aryl allylic alcohols 2i and 2j. | ||
Experimental section
General considerations
IR spectra were recorded on a Bruker Tensor 37 (FT-IR) spectrophotometer. 1H-NMR spectra were recorded on a Bruker Avance 400 (400 MHz) spectrometer at 295 K in CDCl3; chemical shifts (δ in ppm) and coupling constants (J in Hz) are reported in standard fashion with reference to either internal standard tetramethylsilane (TMS) (δH = 0.00 ppm) or CHCl3 (δH = 7.25 ppm). 13C-NMR spectra were recorded on a Bruker Avance 400 (100 MHz) spectrometer at 295 K in CDCl3; chemical shifts (δ in ppm) are reported relative to CHCl3 [δC = 77.00 ppm (central line of triplet)]. In 13C-NMR, the nature of carbons (namely, C, CH, CH2 and CH3) was determined by recording the DEPT-135 spectra, and it is given in parentheses and noted as s = singlet (for C), d = doublet (for CH), t = triplet (for CH2) and q = quartet (for CH3). In 1H-NMR, the following abbreviations were used throughout: s = singlet, d = doublet, t = triplet, q = quartet, qui = quintet, m = multiplet and br. s = broad singlet, septd = septet of doublets. The assignment of signals was confirmed by 1H, 13C CPD and DEPT spectra. High-resolution mass spectra (HR-MS) were recorded on an Agilent 6538 UHD Q-TOF using multimode source. X-ray crystal structure data were obtained using an Oxford Super Nova instrument. All the small scale dry reactions were carried out using the standard syringe-septum technique. Reactions were monitored by TLC on silica gel using a mixture of petroleum ether and ethyl acetate as eluents. Reactions were generally run under an argon or nitrogen atmosphere. All the solvents were distilled prior to use; petroleum ether with a boiling range of 60 to 80 °C, diethyl ether, dichloromethane (DCM), ethyl acetate, THF (with purity 99%), and acetonitrile (with purity 99.9%), which were purchased from locally available commercial sources were used. All aromatic aldehydes (with purity 98%), bromine (with purity 99%), iodine (with purity 99%), Bn(Et)3NCl (with purity 99%), Pd(OAc)2 (with purity 98%), 3-iodoanisole (with purity 99%), 2-bromoiodobenzene (with purity 99%), NaBH4 (with purity 99%), K2CO3 (with purity 99%), and NaHCO3 (with purity 99.5%) were purchased from Sigma-Aldrich, whereas vinylmagnesium bromide (with purity 99%), BF3·Et2O (with purity 48%) and iodobenzene (with purity 99%) were purchased from other commercial sources and used as received. The base NaHCO3 was dried at 150–170 °C over an oil bath. Diethyl ether and toluene were dried over sodium/benzophenone, DCM and DCE were dried over calcium hydride, and acetonitrile was dried over P2O5. Acme's silica gel (60–120 mesh) was used for column chromatography (approximately 20 g per one gram of crude material).ortho-Bromobenzaldehydes 1a–1h except 1a were synthesized from corresponding aromatic aldehydes using a bromination method reported in the literature.17 Among the bromo aryl allylic alcohols, 2a,18 2g13b and 2h13b were reported in literature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 80
10 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) furnished ortho-bromo aryl allylic alcohols 2a–2h (80–92%).
20) furnished ortho-bromo aryl allylic alcohols 2a–2h (80–92%).1-[5-(Benzyloxy)-2-bromophenyl]prop-2-en-1-ol (2b). GP-1 was carried out and the product 2b (2.80 g, 88%) was furnished as a pale yellow liquid. [TLC control Rf(1b) = 0.60, Rf(2b) = 0.40 (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3371, 3032, 2920, 1592, 1571, 1462, 1291, 1380, 1291, 1233, 1163, 1010, 927, 736, 697 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.50–7.28 (m, 6H, Ar-H), 7.19 (d, 1H, J = 2.9 Hz, Ar-H), 6.78 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 5.99 (ddd, 1H, J = 15.6, 10.3 and 5.4 Hz, CH
10, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3371, 3032, 2920, 1592, 1571, 1462, 1291, 1380, 1291, 1233, 1163, 1010, 927, 736, 697 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.50–7.28 (m, 6H, Ar-H), 7.19 (d, 1H, J = 2.9 Hz, Ar-H), 6.78 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 5.99 (ddd, 1H, J = 15.6, 10.3 and 5.4 Hz, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.54 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.40 (td, 1H, J = 15.6 and 1.5 Hz, C
CH2), 5.54 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.40 (td, 1H, J = 15.6 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.22 (td, 1H, J = 10.3 and 1.5 Hz, C
CHaHb), 5.22 (td, 1H, J = 10.3 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.04 (s, 2H, PhCH2O), 2.25 (d, 1H, J = 3.9 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 158.4 (s, Ar-C), 142.5 (s, Ar-C), 138.1 (d, CH
CHaHb), 5.04 (s, 2H, PhCH2O), 2.25 (d, 1H, J = 3.9 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 158.4 (s, Ar-C), 142.5 (s, Ar-C), 138.1 (d, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 136.4 (s, Ar-C), 133.3 (d, Ar-CH), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.5 (d, 2C, Ar-CH), 115.9 (d, Ar-CH), 115.7 (t, CH
CH2), 136.4 (s, Ar-C), 133.3 (d, Ar-CH), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.5 (d, 2C, Ar-CH), 115.9 (d, Ar-CH), 115.7 (t, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 114.2 (d, Ar-CH), 112.9 (s, Ar-C), 73.4 (d, Ar-CHOH), 70.2 (t, PhCH2) ppm. HR-MS (ESI+) m/z calculated for [C16H14BrO]+ = [(M + H)–H2O]+: 301.0223; found 301.0213.
CH2), 114.2 (d, Ar-CH), 112.9 (s, Ar-C), 73.4 (d, Ar-CHOH), 70.2 (t, PhCH2) ppm. HR-MS (ESI+) m/z calculated for [C16H14BrO]+ = [(M + H)–H2O]+: 301.0223; found 301.0213.
1-(2-Bromo-5-methoxyphenyl)prop-2-en-1-ol (2c). GP-1 was carried out and the product 2c (2.20 mg, 92%) was furnished as a pale yellow liquid. [TLC control Rf(1c) = 0.80, Rf(2c) = 0.50 (petroleum ether/ethyl acetate 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3380, 2922, 2851, 1593, 1572, 1468, 1416, 1290, 1233, 1161, 1047, 1013, 928, 807, 771 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.39 (d, 1H, J = 8.8 Hz, Ar-H), 7.07 (d, 1H, J = 3.4 Hz, Ar-H), 6.69 (dd, 1H, J = 8.8 and 3.4 Hz, Ar-H), 5.99 (ddd, 1H, J = 17.1, 10.3 and 5.4 Hz, CH
20, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3380, 2922, 2851, 1593, 1572, 1468, 1416, 1290, 1233, 1161, 1047, 1013, 928, 807, 771 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.39 (d, 1H, J = 8.8 Hz, Ar-H), 7.07 (d, 1H, J = 3.4 Hz, Ar-H), 6.69 (dd, 1H, J = 8.8 and 3.4 Hz, Ar-H), 5.99 (ddd, 1H, J = 17.1, 10.3 and 5.4 Hz, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.53 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.38 (td, 1H, J = 17.1 and 1.5 Hz, C
CH2), 5.53 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.38 (td, 1H, J = 17.1 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.21 (dd, 1H, J = 10.3 and 1.5 Hz, C
CHaHb), 5.21 (dd, 1H, J = 10.3 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 3.78 (s, 3H, Ar-OCH3), 2.34 (br. s, 1H, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 159.3 (s, Ar-C), 142.4 (s, Ar-C), 138.1 (d, CH
CHaHb), 3.78 (s, 3H, Ar-OCH3), 2.34 (br. s, 1H, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 159.3 (s, Ar-C), 142.4 (s, Ar-C), 138.1 (d, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 133.3 (d, Ar-CH), 115.7 (t, CH
CH2), 133.3 (d, Ar-CH), 115.7 (t, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 115.2 (d, Ar-CH), 113.0 (d, Ar-CH), 112.7 (s, Ar-C), 73.4 (d, Ar-CHOH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C10H10BrO]+ = [(M + H)–H2O]+: 224.9910; found 224.9903.
CH2), 115.2 (d, Ar-CH), 113.0 (d, Ar-CH), 112.7 (s, Ar-C), 73.4 (d, Ar-CHOH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C10H10BrO]+ = [(M + H)–H2O]+: 224.9910; found 224.9903.
1-[5-(Benzyloxy)-2-bromo-4-methoxyphenyl]prop-2-en-1-ol (2d). GP-1 was carried out and the product 2d (2.96 g, 85%) was furnished as a brownish viscous liquid. [TLC control Rf(1d) = 0.60, Rf(2d) = 0.30 (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3392, 2933, 2847, 1599, 1497, 1454, 1381, 1251, 1120, 1155, 1120, 1039, 1023, 861, 834, 696, 665 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.37 (t, 2H, J = 7.3 Hz, Ar-H), 7.31 (ddd, 1H, J = 7.3 and 6.8 Hz, Ar-H), 7.03 (s, 1H, Ar-H), 7.02 (s, 1H, Ar-H), 5.98 (ddd, 1H, J = 15.6, 10.3 and 4.9 Hz, CH
10, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3392, 2933, 2847, 1599, 1497, 1454, 1381, 1251, 1120, 1155, 1120, 1039, 1023, 861, 834, 696, 665 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.37 (t, 2H, J = 7.3 Hz, Ar-H), 7.31 (ddd, 1H, J = 7.3 and 6.8 Hz, Ar-H), 7.03 (s, 1H, Ar-H), 7.02 (s, 1H, Ar-H), 5.98 (ddd, 1H, J = 15.6, 10.3 and 4.9 Hz, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.51 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.38 (td, 1H, J = 15.6 and 1.5 Hz, C
CH2), 5.51 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.38 (td, 1H, J = 15.6 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.20 (td, 1H, J = 10.3 and 1.5 Hz, C
CHaHb), 5.20 (td, 1H, J = 10.3 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.09 (s, 2H, PhCH2O), 3.38 (s, 3H, Ar-OCH3), 2.29 (d, 1H, J = 2.9 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 149.4 (s, Ar-C), 148.1 (s, Ar-C), 138.5 (d, CH
CHaHb), 5.09 (s, 2H, PhCH2O), 3.38 (s, 3H, Ar-OCH3), 2.29 (d, 1H, J = 2.9 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 149.4 (s, Ar-C), 148.1 (s, Ar-C), 138.5 (d, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 136.3 (s, Ar-C), 134.0 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.0 (d, Ar-CH), 127.3 (d, 2C, Ar-CH), 117.6 (d, Ar-CH), 115.3 (t, CH
CH2), 136.3 (s, Ar-C), 134.0 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.0 (d, Ar-CH), 127.3 (d, 2C, Ar-CH), 117.6 (d, Ar-CH), 115.3 (t, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 112.1 (s, Ar-C), 110.7 (d, Ar-CH), 73.3 (d, Ar-CHOH), 71.2 (t, PhCH2), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16BrO2]+ = [(M + H)–H2O]+: 331.0328; found 331.0332.
CH2), 112.1 (s, Ar-C), 110.7 (d, Ar-CH), 73.3 (d, Ar-CHOH), 71.2 (t, PhCH2), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16BrO2]+ = [(M + H)–H2O]+: 331.0328; found 331.0332.
1-[4-(Benzyloxy)-2-bromo-5-methoxyphenyl]prop-2-en-1-ol (2e). GP-1 was carried out and the product 2e (2.79 g, 80%) was furnished as a brownish viscous liquid. [TLC control Rf(1e) = 0.60, Rf(2e) = 0.30 (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3404, 3032, 3008, 2932, 1600, 1502, 1502, 1439, 1379, 1257, 1156, 1120, 1029, 925, 863, 777 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42 (d, 2H, J = 7.3 Hz, Ar-H), 7.35 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.29 (t, 1H, J = 7.3 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.00 (s, 1H, Ar-H), 5.90 (ddd, 1H, J = 15.6, 10.3 and 4.9 Hz, CH
10, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3404, 3032, 3008, 2932, 1600, 1502, 1502, 1439, 1379, 1257, 1156, 1120, 1029, 925, 863, 777 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 7.42 (d, 2H, J = 7.3 Hz, Ar-H), 7.35 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.29 (t, 1H, J = 7.3 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.00 (s, 1H, Ar-H), 5.90 (ddd, 1H, J = 15.6, 10.3 and 4.9 Hz, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.49 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.35 (td, 1H, J = 15.6 and 1.5 Hz, C
CH2), 5.49 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.35 (td, 1H, J = 15.6 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.31 (td, 1H, J = 10.3 and 1.5 Hz, C
CHaHb), 5.31 (td, 1H, J = 10.3 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.11 (d, 1H, J = 12.2 Hz, PhCHaHbO), 5.10 (d, 1H, J = 12.2 Hz, PhCHaHbO), 3.85 (s, 3H, Ar-OCH3), 2.10 (d, 1H, J = 2.4 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 149.6 (s, Ar-C), 147.8 (s, Ar-C), 138.4 (d, CH
CHaHb), 5.11 (d, 1H, J = 12.2 Hz, PhCHaHbO), 5.10 (d, 1H, J = 12.2 Hz, PhCHaHbO), 3.85 (s, 3H, Ar-OCH3), 2.10 (d, 1H, J = 2.4 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 149.6 (s, Ar-C), 147.8 (s, Ar-C), 138.4 (d, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 136.5 (s, Ar-C), 133.4 (s, Ar-C), 128.5 (d, 2C, Ar-CH), 128.0 (d, Ar-CH), 127.6 (d, 2C, Ar-CH), 115.7 (d, Ar-CH), 115.3 (t, CH
CH2), 136.5 (s, Ar-C), 133.4 (s, Ar-C), 128.5 (d, 2C, Ar-CH), 128.0 (d, Ar-CH), 127.6 (d, 2C, Ar-CH), 115.7 (d, Ar-CH), 115.3 (t, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 113.1 (s, Ar-C), 112.9 (d, Ar-CH), 73.2 (d, Ar-CHOH), 71.1 (t, PhCH2), 56.2 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16BrO2]+ = [(M + H)–H2O]+: 331.0328; found 331.0334.
CH2), 113.1 (s, Ar-C), 112.9 (d, Ar-CH), 73.2 (d, Ar-CHOH), 71.1 (t, PhCH2), 56.2 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H16BrO2]+ = [(M + H)–H2O]+: 331.0328; found 331.0334.
1-(6-Bromo-1,3-benzodioxol-5-yl)prop-2-en-1-ol (2f). GP-1 was carried out and the product 2f (2.0 g, 80%) was furnished as a colorless viscous liquid. [TLC control Rf(1f) = 0.60, Rf(2f) = 0.50 (petroleum ether/ethyl acetate 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3346, 2897, 1501, 1471, 1407, 1230, 1107, 1035, 930, 840, 798 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 6.98 (s, 1H, Ar-H), 6.96 (s, 1H, Ar-H), 5.95 (d, 1H, J = 5.4 Hz, OCHaHbO), 5.94 (d, 1H, J = 5.4 Hz, OCHaHbO), 5.93 (ddd, 1H, J = 15.6, 10.3 and 5.4 Hz, CH
20, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3346, 2897, 1501, 1471, 1407, 1230, 1107, 1035, 930, 840, 798 cm−1. 1H NMR (CDCl3, 400 MHz): δ = 6.98 (s, 1H, Ar-H), 6.96 (s, 1H, Ar-H), 5.95 (d, 1H, J = 5.4 Hz, OCHaHbO), 5.94 (d, 1H, J = 5.4 Hz, OCHaHbO), 5.93 (ddd, 1H, J = 15.6, 10.3 and 5.4 Hz, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.51 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.37 (td, 1H, J = 15.6 and 1.5 Hz, C
CH2), 5.51 (d, 1H, J = 5.4 Hz, ArCH–OH), 5.37 (td, 1H, J = 15.6 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 5.20 (td, 1H, J = 10.3 and 1.5 Hz, C
CHaHb), 5.20 (td, 1H, J = 10.3 and 1.5 Hz, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHaHb), 2.26 (d, 1H, J = 2.9 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 147.8 (s, Ar-C), 147.7 (s, Ar-C), 138.4 (d, CH
CHaHb), 2.26 (d, 1H, J = 2.9 Hz, OH) ppm. 13C NMR (CDCl3, 100 MHz): δ = 147.8 (s, Ar-C), 147.7 (s, Ar-C), 138.4 (d, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 134.8 (s, Ar-C), 115.3 (t, CH
CH2), 134.8 (s, Ar-C), 115.3 (t, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 112.8 (s, Ar-C), 112.5 (d, Ar-CH), 107.7 (d, Ar-CH), 101.7 (t, OCH2O), 73.3 (d, Ar-CHOH) ppm. HR-MS (ESI+) m/z calculated for [C10H9BrNaO3]+ = [M + Na]+: 278.9627; found 278.9639.
CH2), 112.8 (s, Ar-C), 112.5 (d, Ar-CH), 107.7 (d, Ar-CH), 101.7 (t, OCH2O), 73.3 (d, Ar-CHOH) ppm. HR-MS (ESI+) m/z calculated for [C10H9BrNaO3]+ = [M + Na]+: 278.9627; found 278.9639.
1-[(E)-2-(2-Bromophenyl)vinyl]-1,3-dihydro-2-benzofuran (6aa). GP-2 was carried out and the product 6aa (83 mg, 55%) was furnished as a yellow viscous liquid. [TLC control Rf(1a) = 0.80, Rf(2a) = 0.70 and Rf(6aa) = 0.85 (petroleum ether/ethyl acetate 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2852, 1588, 1465, 1437, 1357, 1284, 1246, 1158, 1122, 1107, 1021, 963, 747, 698, 665 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.53 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.50 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.35–7.15 (m, 5H, Ar-H), 7.10 (d, 1H, J = 15.6 Hz, ArCH
5, UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2852, 1588, 1465, 1437, 1357, 1284, 1246, 1158, 1122, 1107, 1021, 963, 747, 698, 665 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.53 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.50 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.35–7.15 (m, 5H, Ar-H), 7.10 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.08 (ddd, 1H, J = 9.3, 7.8 and 1.5 Hz, Ar-H), 6.21 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 7.08 (ddd, 1H, J = 9.3, 7.8 and 1.5 Hz, Ar-H), 6.21 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.80 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.80 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.22 (d, 1H, J = 11.7 Hz, PhCHaHbOCHCH
CH], 5.22 (d, 1H, J = 11.7 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.14 (d, 1H, J = 11.7 Hz, PhCHaHbOCHCH
CH), 5.14 (d, 1H, J = 11.7 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 140.6 (s, Ar-C), 139.1 (s, Ar-C), 136.4 (s, Ar-C), 132.9 (d, Ar-CH), 132.0 (d, Ar-CH), 130.5 (d, Ar-CH–CH
CH) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 140.6 (s, Ar-C), 139.1 (s, Ar-C), 136.4 (s, Ar-C), 132.9 (d, Ar-CH), 132.0 (d, Ar-CH), 130.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 129.1 (d, Ar-CH–CH
CH-Ar), 129.1 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 127.8 (d, Ar-CH), 127.5 (d, Ar-CH), 127.4 (d, Ar-CH), 127.3 (d, Ar-CH), 123.8 (s, Ar-C), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 85.0 (d, Ph-CHCH
CH-Ar), 127.8 (d, Ar-CH), 127.5 (d, Ar-CH), 127.4 (d, Ar-CH), 127.3 (d, Ar-CH), 123.8 (s, Ar-C), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 85.0 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.9 (t, Ph-CH2OCHCH
CH), 72.9 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) ppm. HR-MS (ESI+): m/z calculated for [C16H13BrNaO]+ = [M + Na]+: 323.0042; found 323.0041.
CH) ppm. HR-MS (ESI+): m/z calculated for [C16H13BrNaO]+ = [M + Na]+: 323.0042; found 323.0041.
1-{(E)-2-[5-(Benzyloxy)-2-bromophenyl]vinyl}-1,3-dihydro-2-benzofuran (6ab). GP-2 was carried out and the product 6ab (92 mg, 45%) was furnished as a pale yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, Rf(1a) = 0.80, Rf(2b) = 0.50 and Rf(6ab) = 0.65 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2852, 1590, 1563, 1459, 1286, 1238, 1173, 1028, 1013, 963, 739, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.41 (d, 1H, J = 8.3 Hz, Ar-H), 7.39–7.20 (m, 8H, Ar-H), 7.19 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 7.14 (d, 1H, J = 2.9 Hz, Ar-H), 7.05 (d, 1H, J = 15.6 Hz, ArCH
5, Rf(1a) = 0.80, Rf(2b) = 0.50 and Rf(6ab) = 0.65 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2852, 1590, 1563, 1459, 1286, 1238, 1173, 1028, 1013, 963, 739, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.41 (d, 1H, J = 8.3 Hz, Ar-H), 7.39–7.20 (m, 8H, Ar-H), 7.19 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 7.14 (d, 1H, J = 2.9 Hz, Ar-H), 7.05 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.73 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 6.19 (dd, 1H, J = 15.6 and 7.3 Hz, ArCH
CH), 6.73 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 6.19 (dd, 1H, J = 15.6 and 7.3 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.80 [d, 1H, J = 7.3 Hz, PhCH(O)CH
CH), 5.80 [d, 1H, J = 7.3 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.22 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.22 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.13 (dd, 1H, J = 12.2 and 1.0 Hz, PhCHaHbOCHCH
CH), 5.13 (dd, 1H, J = 12.2 and 1.0 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.98 (s, 2H, PhCH2O) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 158.0 (s, Ar-C), 140.5 (s, Ar-C), 139.1 (s, Ar-C), 137.1 (s, Ar-C), 136.4 (s, Ar-C), 133.4 (d, Ar-CH), 132.1 (d, Ar-CH), 130.5 (d, Ar-CH), 128.5 (d, 2C, Ar-CH), 128.0 (d, Ar-CH–CH
CH), 4.98 (s, 2H, PhCH2O) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 158.0 (s, Ar-C), 140.5 (s, Ar-C), 139.1 (s, Ar-C), 137.1 (s, Ar-C), 136.4 (s, Ar-C), 133.4 (d, Ar-CH), 132.1 (d, Ar-CH), 130.5 (d, Ar-CH), 128.5 (d, 2C, Ar-CH), 128.0 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 127.8 (d, Ar-CH–CH
CH-Ar), 127.8 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 127.5 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 116.2 (d, Ar-CH), 114.8 (s, Ar-C), 113.3 (d, Ar-CH), 84.8 (d, Ph-CHCH
CH-Ar), 127.5 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 116.2 (d, Ar-CH), 114.8 (s, Ar-C), 113.3 (d, Ar-CH), 84.8 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ph-CH2OCHCH
CH), 72.8 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) 70.1 (t, PhCH2O) ppm. HR-MS (ESI+): m/z calculated for [C23H1879BrO]+ = [(M + H)–H2O]+: 389.0536; found 389.0545 and [C23H1881BrO]+ = [(M + H)–H2O]+: 391.0515; found 391.0529.
CH) 70.1 (t, PhCH2O) ppm. HR-MS (ESI+): m/z calculated for [C23H1879BrO]+ = [(M + H)–H2O]+: 389.0536; found 389.0545 and [C23H1881BrO]+ = [(M + H)–H2O]+: 391.0515; found 391.0529.
1-[(E)-2-(2-Bromo-5-methoxyphenyl)vinyl]-1,3-dihydro-2-benzo furan (6ac). GP-2 was carried out and the product 6ac (80 mg, 48%) was furnished as a pale brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, Rf(1a) = 0.75, Rf(2c) = 0.35 and Rf(6ac) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2959, 2929, 1592, 1571, 1464, 1287, 1236, 1161, 1014, 802, 754, 733, 599 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.35 (d, 1H, J = 8.8 Hz, Ar-H), 7.30–7.05 (m, 4H, Ar-H), 7.01 (d, 1H, J = 15.5 Hz, ArCH
5, Rf(1a) = 0.75, Rf(2c) = 0.35 and Rf(6ac) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2959, 2929, 1592, 1571, 1464, 1287, 1236, 1161, 1014, 802, 754, 733, 599 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.35 (d, 1H, J = 8.8 Hz, Ar-H), 7.30–7.05 (m, 4H, Ar-H), 7.01 (d, 1H, J = 15.5 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.97 (d, 1H, J = 2.2 Hz, Ar-H), 6.62 (dd, 1H, J = 8.7 and 3.0 Hz, Ar-H), 6.13 (dd, 1H, J = 15.5 and 7.5 Hz, ArCH
CH), 6.97 (d, 1H, J = 2.2 Hz, Ar-H), 6.62 (dd, 1H, J = 8.7 and 3.0 Hz, Ar-H), 6.13 (dd, 1H, J = 15.5 and 7.5 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.74 [d, 1H, J = 7.5 Hz, PhCH(O)CH
CH), 5.74 [d, 1H, J = 7.5 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.16 (dd, 1H, J = 12.3 and 2.2 Hz, PhCHaHbOCHCH
CH], 5.16 (dd, 1H, J = 12.3 and 2.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.08 (d, 1H, J = 12.3 Hz, PhCHaHbOCHCH
CH), 5.08 (d, 1H, J = 12.3 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.68 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 158.9 (s, Ar-C), 140.5 (s, Ar-C), 139.1 (s, Ar-C), 140.0 (s, Ar-C), 133.4 (d, Ar-CH), 132.0 (d, Ar-CH), 130.7 (d, Ar-CH), 127.8 (d, Ar-CH–CH
CH), 3.68 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 158.9 (s, Ar-C), 140.5 (s, Ar-C), 139.1 (s, Ar-C), 140.0 (s, Ar-C), 133.4 (d, Ar-CH), 132.0 (d, Ar-CH), 130.7 (d, Ar-CH), 127.8 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 127.5 (d, Ar-CH–CH
CH-Ar), 127.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 115.7 (d, Ar-CH), 114.5 (s, Ar-C), 112.0 (d, Ar-CH), 84.9 (d, Ph-CHCH
CH-Ar), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 115.7 (d, Ar-CH), 114.5 (s, Ar-C), 112.0 (d, Ar-CH), 84.9 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.9 (t, Ph-CH2OCHCH
CH), 72.9 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C17H14BrO]+ = [(M + H)–H2O]+: 313.0223; found 313.0212, [C17H1481BrO]+ = [(M + H)–H2O]+:315.0202; found 315.0189 and [C17H19BrNO2]+ = [M + NH4]+: 348.0594; found 348.0587.
CH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C17H14BrO]+ = [(M + H)–H2O]+: 313.0223; found 313.0212, [C17H1481BrO]+ = [(M + H)–H2O]+:315.0202; found 315.0189 and [C17H19BrNO2]+ = [M + NH4]+: 348.0594; found 348.0587.
1-{(E)-2-[5-(Benzyloxy)-2-bromo-4-methoxyphenyl]vinyl}-1,3-dihydro-2-benzofuran (6ad). GP-2 was carried out and the product 6ad (92 mg, 42%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1a) = 0.80, Rf(2d) = 0.20 and Rf(6ad) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2918, 2850, 1595, 1502, 1461, 1385, 1260, 1200, 1166, 1024, 861, 750, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.43 (d, 2H, J = 7.3 Hz, Ar-H), 7.38 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.35–7.25 (m, 4H, Ar-H), 7.22 (dd, 1H, J = 7.8 and 2.0 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.04 (s, 1H, Ar-H), 7.03 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1a) = 0.80, Rf(2d) = 0.20 and Rf(6ad) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2918, 2850, 1595, 1502, 1461, 1385, 1260, 1200, 1166, 1024, 861, 750, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.43 (d, 2H, J = 7.3 Hz, Ar-H), 7.38 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.35–7.25 (m, 4H, Ar-H), 7.22 (dd, 1H, J = 7.8 and 2.0 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.04 (s, 1H, Ar-H), 7.03 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.13 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.13 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.81 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.81 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.25 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.25 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.14 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH
CH), 5.14 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.10 (s, 2H, PhCH2O) 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.1 (s, Ar-C), 148.6 (s, Ar-C), 140.7 (s, Ar-C), 139.2 (s, Ar-C), 136.2 (s, Ar-C), 130.7 (d, Ar-CH–CH
CH), 5.10 (s, 2H, PhCH2O) 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.1 (s, Ar-C), 148.6 (s, Ar-C), 140.7 (s, Ar-C), 139.2 (s, Ar-C), 136.2 (s, Ar-C), 130.7 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.0 (d, Ar-CH–CH
CH-Ar), 130.0 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.8 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.8 (d, Ar-CH), 127.5 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 117.6 (d, Ar-CH), 114.4 (s, Ar-C), 109.6 (d, Ar-CH), 85.2 (d, Ph-CHCH
CH-Ar), 128.8 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.8 (d, Ar-CH), 127.5 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 117.6 (d, Ar-CH), 114.4 (s, Ar-C), 109.6 (d, Ar-CH), 85.2 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ph-CH2OCHCH
CH), 72.8 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 71.1 (t, PhCH2O), 56.1 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C24H21BrNaO3]+ = [M + Na]+: 459.0566; found 459.0583 and [C24H21BrNaO3]+ = [M + Na]+: 461.0546; found 461.0561.
CH), 71.1 (t, PhCH2O), 56.1 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C24H21BrNaO3]+ = [M + Na]+: 459.0566; found 459.0583 and [C24H21BrNaO3]+ = [M + Na]+: 461.0546; found 461.0561.
1-{(E)-2-[4-(Benzyloxy)-2-bromo-5-methoxyphenyl]vinyl}-1,3-dihydro-2-benzofuran (6ae). GP-2 was carried out and the product 6ae (100 mg, 45%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1a) = 0.80, Rf(2e) = 0.20 and Rf(6ae) = 0.35 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2957, 2920, 2851, 1503, 1462, 1441, 1379, 1261, 1206, 1163, 1026, 743 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.40 (d, 2H, J = 6.8 Hz, Ar-H), 7.34 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.31–7.24 (m, 4H, Ar-H), 7.20 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 7.08 (s, 1H, J = 9.3 Hz, Ar-H), 7.03 (s, 1H, J = 9.3 Hz, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1a) = 0.80, Rf(2e) = 0.20 and Rf(6ae) = 0.35 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2957, 2920, 2851, 1503, 1462, 1441, 1379, 1261, 1206, 1163, 1026, 743 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.40 (d, 2H, J = 6.8 Hz, Ar-H), 7.34 (dd, 2H, J = 7.3 and 6.8 Hz, Ar-H), 7.31–7.24 (m, 4H, Ar-H), 7.20 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 7.08 (s, 1H, J = 9.3 Hz, Ar-H), 7.03 (s, 1H, J = 9.3 Hz, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.02 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.02 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH
CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.06 (s, 2H, PhCH2O) 3.86 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 150.2 (s, Ar-C), 147.7 (s, Ar-C), 140.8 (s, Ar-C), 139.2 (s, Ar-C), 136.5 (s, Ar-C), 130.6 (d, Ar-CH–CH
CH), 5.06 (s, 2H, PhCH2O) 3.86 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 150.2 (s, Ar-C), 147.7 (s, Ar-C), 140.8 (s, Ar-C), 139.2 (s, Ar-C), 136.5 (s, Ar-C), 130.6 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 129.9 (d, Ar-CH–CH
CH-Ar), 129.9 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.6 (d, 2C, Ar-CH), 128.4 (s, Ar-C), 128.1 (d, Ar-CH), 127.8 (d, Ar-CH), 127.6 (d, 2C, Ar-CH), 127.5 (d, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 115.8 (d, Ar-CH), 115.2 (s, Ar-C), 112.1 (d, Ar-CH), 85.2 (d, Ph-CHCH
CH-Ar), 128.6 (d, 2C, Ar-CH), 128.4 (s, Ar-C), 128.1 (d, Ar-CH), 127.8 (d, Ar-CH), 127.6 (d, 2C, Ar-CH), 127.5 (d, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 115.8 (d, Ar-CH), 115.2 (s, Ar-C), 112.1 (d, Ar-CH), 85.2 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.9 (t, Ph-CH2OCHCH
CH), 72.9 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 71.3 (t, PhCH2O), 56.2 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C24H22BrO3]+ = [M + H]+: 437.0747; found 437.0735 and [C24H2281BrO3]+ = [M + H]+: 439.0726; found 439.0732.
CH), 71.3 (t, PhCH2O), 56.2 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C24H22BrO3]+ = [M + H]+: 437.0747; found 437.0735 and [C24H2281BrO3]+ = [M + H]+: 439.0726; found 439.0732.
5-Bromo-6-[(E)-2-(1,3-dihydro-2-benzofuran-1-yl)vinyl]-1,3-benzodioxole (6af). GP-2 was carried out and the product 6af (90 mg, 52%) was furnished as a pale yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1a) = 0.80, Rf(2f) = 0.30 and Rf(6af) = 0.65 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2901, 2852, 1502, 1474, 1412, 1247, 1229, 1116, 1034, 978, 961, 933, 863, 838, 750 cm−1.1H-NMR (CDCl3, 400 MHz): δ = 7.35–7.23 (m, 3H, Ar-H), 7.19 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 7.03 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1a) = 0.80, Rf(2f) = 0.30 and Rf(6af) = 0.65 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2901, 2852, 1502, 1474, 1412, 1247, 1229, 1116, 1034, 978, 961, 933, 863, 838, 750 cm−1.1H-NMR (CDCl3, 400 MHz): δ = 7.35–7.23 (m, 3H, Ar-H), 7.19 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 7.03 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.99 (d, 2H, J = 2.4 Hz, Ar-H), 6.07 (dd, 1H, J = 15.5 and 7.8 Hz, ArCH
CH), 6.99 (d, 2H, J = 2.4 Hz, Ar-H), 6.07 (dd, 1H, J = 15.5 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.94 (s, 2H, OCH2O), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.94 (s, 2H, OCH2O), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.22 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.22 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH
CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 148.1 (s, Ar-C), 147.6 (s, Ar-C), 140.7 (s, Ar-C), 139.1 (s, Ar-C), 130.5 (d, Ar-CH–CH
CH) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 148.1 (s, Ar-C), 147.6 (s, Ar-C), 140.7 (s, Ar-C), 139.1 (s, Ar-C), 130.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.3 (d, Ar-CH–CH
CH-Ar), 130.3 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 129.6 (s, Ar-C), 127.8 (d, Ar-CH), 127.4 (d, Ar-CH), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 115.0 (s, Ar-C), 112.6 (d, Ar-CH), 106.4 (d, Ar-CH), 101.7 (d, Ar-CH), 85.0 (d, Ph-CHCH
CH-Ar), 129.6 (s, Ar-C), 127.8 (d, Ar-CH), 127.4 (d, Ar-CH), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 115.0 (s, Ar-C), 112.6 (d, Ar-CH), 106.4 (d, Ar-CH), 101.7 (d, Ar-CH), 85.0 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ph-CH2OCHCH
CH), 72.8 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) ppm. HR-MS (ESI+): m/z calculated for [C17H13BrNaO3]+ = [M + Na]+: 366.9940; found 366.9938 and [C17H1381BrNaO3]+ = [M + Na]+: 368.9920; found 368.9918.
CH) ppm. HR-MS (ESI+): m/z calculated for [C17H13BrNaO3]+ = [M + Na]+: 366.9940; found 366.9938 and [C17H1381BrNaO3]+ = [M + Na]+: 368.9920; found 368.9918.
1-[(E)-2-(2-Bromo-4,5-dimethoxyphenyl)vinyl]-1,3-dihydro-2-benzofuran (6ag). GP-2 was carried out and the product 6ag (78 mg, 43%) was furnished as a yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1a) = 0.80, Rf(2g) = 0.15 and Rf(6ag) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2928, 2847, 1502, 1462, 1439, 1380, 1256, 1160, 1024, 751 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.30–7.15 (m, 4H, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1a) = 0.80, Rf(2g) = 0.15 and Rf(6ag) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2928, 2847, 1502, 1462, 1439, 1380, 1256, 1160, 1024, 751 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.30–7.15 (m, 4H, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.99 (s, 1H, Ar-H), 6.98 (s, 1H, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.99 (s, 1H, Ar-H), 6.98 (s, 1H, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.21 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.21 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH
CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.83 (s, 3H, Ar-OCH3), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.4 (s, Ar-C), 148.4 (s, Ar-C), 140.6 (s, Ar-C), 139.1 (s, Ar-C), 130.6 (d, Ar-CH–CH
CH), 3.83 (s, 3H, Ar-OCH3), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.4 (s, Ar-C), 148.4 (s, Ar-C), 140.6 (s, Ar-C), 139.1 (s, Ar-C), 130.6 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 129.8 (d, Ar-CH–CH
CH-Ar), 129.8 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.2 (s, Ar-C), 127.7 (d, Ar-CH), 127.4 (d, Ar-CH), 122.0 (d, Ar-CH), 121.0 (d, Ar-CH), 115.2 (d, Ar-CH), 114.5 (s, Ar-C), 109.0 (d, Ar-CH), 85.2 (d, Ph-CHCH
CH-Ar), 128.2 (s, Ar-C), 127.7 (d, Ar-CH), 127.4 (d, Ar-CH), 122.0 (d, Ar-CH), 121.0 (d, Ar-CH), 115.2 (d, Ar-CH), 114.5 (s, Ar-C), 109.0 (d, Ar-CH), 85.2 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ph-CH2OCHCH
CH), 72.8 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 56.0 (q, Ar-OCH3), 56.9 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C18H17BrNaO3]+ = [M + Na]+: 383.0253; found 383.0254.
CH), 56.0 (q, Ar-OCH3), 56.9 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C18H17BrNaO3]+ = [M + Na]+: 383.0253; found 383.0254.
1-[(E)-2-(2-Bromo-3,4,5-trimethoxyphenyl)vinyl]-1,3-dihydro-2-benzofuran (6ah). GP-2 was carried out and the product 6ah (90 mg, 46%) was furnished as a yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1a) = 0.80, Rf(2h) = 0.10 and Rf(6ah) = 0.25 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2851, 1559, 1480, 1426, 1391, 1325, 1201, 1166, 1106, 1009, 926, 753 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.45–7.15 (m, 4H, Ar-H), 7.10 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1a) = 0.80, Rf(2h) = 0.10 and Rf(6ah) = 0.25 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2851, 1559, 1480, 1426, 1391, 1325, 1201, 1166, 1106, 1009, 926, 753 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.45–7.15 (m, 4H, Ar-H), 7.10 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.87 (s, 1H, Ar-H), 6.13 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.87 (s, 1H, Ar-H), 6.13 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.81 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.81 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.14 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH
CH), 5.14 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.88 (s, 3H, Ar-OCH3), 3.87 (s, 3H, Ar-OCH3), 3.82 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 152.7 (s, Ar-C), 150.8 (s, Ar-C), 143.0 (s, Ar-C), 140.6 (s, Ar-C), 139.1 (s, Ar-C), 131.9 (s, Ar-C), 131.2 (d, Ar-CH–CH
CH), 3.88 (s, 3H, Ar-OCH3), 3.87 (s, 3H, Ar-OCH3), 3.82 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 152.7 (s, Ar-C), 150.8 (s, Ar-C), 143.0 (s, Ar-C), 140.6 (s, Ar-C), 139.1 (s, Ar-C), 131.9 (s, Ar-C), 131.2 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.9 (d, Ar-CH–CH
CH-Ar), 130.9 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 127.8 (d, Ar-CH), 127.5 (d, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 110.8 (s, Ar-C), 105.6 (d, Ar-CH), 85.1 (d, Ph-CHCH
CH-Ar), 127.8 (d, Ar-CH), 127.5 (d, Ar-CH), 122.1 (d, Ar-CH), 121.1 (d, Ar-CH), 110.8 (s, Ar-C), 105.6 (d, Ar-CH), 85.1 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.9 (t, Ph-CH2OCHCH
CH), 72.9 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 61.1 (q, Ar-OCH3), 61.0 (q, Ar-OCH3), 56.1 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C19H18BrO3]+ = [(M + H)–H2O]+: 373.0434; found 373.0416 and [C19H1881BrO3]+ = [(M + H)–H2O]+: 375.0413; found 375.0401.
CH), 61.1 (q, Ar-OCH3), 61.0 (q, Ar-OCH3), 56.1 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C19H18BrO3]+ = [(M + H)–H2O]+: 373.0434; found 373.0416 and [C19H1881BrO3]+ = [(M + H)–H2O]+: 375.0413; found 375.0401.
5-(Benzyloxy)-1-{(E)-2-[5-(benzyloxy)-2-bromo-4-methoxy phenyl]vinyl}-1,3-dihydro-2-benzofuran (6bd). GP-2 was carried out and the product 6bd (111 mg, 41%) was furnished as a yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1b) = 0.70, Rf(2d) = 0.30 and Rf(6bd) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2956, 2922, 2852, 1600, 1500, 1455, 1383, 1260, 1166, 1025, 737, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.50–7.25 (m, 10H, Ar-H), 7.11 (d, 1H, J = 7.8 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.05 (d, 1H, J = 8.3 Hz, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1b) = 0.70, Rf(2d) = 0.30 and Rf(6bd) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2956, 2922, 2852, 1600, 1500, 1455, 1383, 1260, 1166, 1025, 737, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.50–7.25 (m, 10H, Ar-H), 7.11 (d, 1H, J = 7.8 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.05 (d, 1H, J = 8.3 Hz, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.92 (d, 1H, J = 7.8 Hz, Ar-H), 6.87 (s, 1H, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.92 (d, 1H, J = 7.8 Hz, Ar-H), 6.87 (s, 1H, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.18 (dd, 1H, J = 12.7 and 2.0 Hz, ArCHaHbOCHCH
CH], 5.18 (dd, 1H, J = 12.7 and 2.0 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.12 (d, 1H, J = 12.7 Hz, ArCHaHbOCHCH
CH), 5.12 (d, 1H, J = 12.7 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.10 (s, 2H, PhCH2O), 5.08 (s, 2H, PhCH2O), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.1 (s, Ar-C), 149.1 (s, Ar-C), 148.6 (s, Ar-C), 140.9 (s, Ar-C), 136.8 (s, Ar-C), 136.2 (s, Ar-C), 133.0 (s, Ar-C), 130.4 (d, Ar-CH–CH
CH), 5.10 (s, 2H, PhCH2O), 5.08 (s, 2H, PhCH2O), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.1 (s, Ar-C), 149.1 (s, Ar-C), 148.6 (s, Ar-C), 140.9 (s, Ar-C), 136.8 (s, Ar-C), 136.2 (s, Ar-C), 133.0 (s, Ar-C), 130.4 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.3 (d, Ar-CH–CH
CH-Ar), 130.3 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.8 (s, Ar-C), 128.6 (d, 3C, Ar-CH), 128.1 (d, Ar-CH), 128.0 (d, Ar-CH), 127.4 (d, 5C, Ar-CH), 122.9 (d, Ar-CH), 117.6 (d, Ar-CH), 114.6 (d, Ar-CH), 114.3 (s, Ar-C), 109.6 (d, Ar-CH), 107.3 (d, Ar-CH), 84.9 (d, Ar-CHCH
CH-Ar), 128.8 (s, Ar-C), 128.6 (d, 3C, Ar-CH), 128.1 (d, Ar-CH), 128.0 (d, Ar-CH), 127.4 (d, 5C, Ar-CH), 122.9 (d, Ar-CH), 117.6 (d, Ar-CH), 114.6 (d, Ar-CH), 114.3 (s, Ar-C), 109.6 (d, Ar-CH), 107.3 (d, Ar-CH), 84.9 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.7 (t, Ar-CH2OCHCH
CH), 72.7 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 71.1 (t, PhCH2O), 70.3 (t, PhCH2O), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C31H28BrO4]+ = [M + H]+: 543.1165; found 543.1140 and [C31H2881BrO4]+ = [M + H]+: 545.1145; found 545.1130, [C31H27BrNaO4]+ = [M + Na]+: 565.0985; found 565.0959 and [C31H2781BrNaO4]+ = [M + Na]+: 567.0964; found 567.0977.
CH), 71.1 (t, PhCH2O), 70.3 (t, PhCH2O), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C31H28BrO4]+ = [M + H]+: 543.1165; found 543.1140 and [C31H2881BrO4]+ = [M + H]+: 545.1145; found 545.1130, [C31H27BrNaO4]+ = [M + Na]+: 565.0985; found 565.0959 and [C31H2781BrNaO4]+ = [M + Na]+: 567.0964; found 567.0977.
5-(Benzyloxy)-1-{(E)-2-[4-(benzyloxy)-2-bromo-5-methoxy phenyl]vinyl}-1,3-dihydro-2-benzofuran (6be). GP-2 was carried out and the product 6be (119 mg, 44%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1b) = 0.70, Rf(2e) = 0.30 and Rf(6be) = 0.55 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1600, 1502, 1455, 1439, 1380, 1259, 1163, 1026, 737, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.50–7.20 (m, 10H, Ar-H), 7.09 (s, 1H, Ar-H), 7.05 (d, 1H, J = 7.8 Hz, Ar-H), 7.03 (s, 1H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1b) = 0.70, Rf(2e) = 0.30 and Rf(6be) = 0.55 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1600, 1502, 1455, 1439, 1380, 1259, 1163, 1026, 737, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.50–7.20 (m, 10H, Ar-H), 7.09 (s, 1H, Ar-H), 7.05 (d, 1H, J = 7.8 Hz, Ar-H), 7.03 (s, 1H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.91 (dd, 1H, J = 8.3 and 2.0 Hz, Ar-H), 6.86 (d, 1H, J = 2.0 Hz, Ar-H), 6.09 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.91 (dd, 1H, J = 8.3 and 2.0 Hz, Ar-H), 6.86 (d, 1H, J = 2.0 Hz, Ar-H), 6.09 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.74 [d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.74 [d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.17 (dd, 1H, J = 12.2 and 2.0 Hz, ArCHaHbOCHCH
CH], 5.17 (dd, 1H, J = 12.2 and 2.0 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.10 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH
CH), 5.10 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.07 (s, 2H, PhCH2O), 5.07 (s, 2H, PhCH2O), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.1 (s, Ar-C), 150.2 (s, Ar-C), 148.6 (s, Ar-C), 140.9 (s, Ar-C), 136.8 (s, Ar-C), 136.2 (s, Ar-C), 133.1 (s, Ar-C), 130.5 (d, Ar-CH–CH
CH), 5.07 (s, 2H, PhCH2O), 5.07 (s, 2H, PhCH2O), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.1 (s, Ar-C), 150.2 (s, Ar-C), 148.6 (s, Ar-C), 140.9 (s, Ar-C), 136.8 (s, Ar-C), 136.2 (s, Ar-C), 133.1 (s, Ar-C), 130.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.2 (d, Ar-CH–CH
CH-Ar), 130.2 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.7 (d, 2C, Ar-CH), 128.6 (d, 2C, Ar-CH), 128.1 (s, Ar-C), 128.0 (d, 2C, Ar-CH), 127.4 (d, 2C, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.9 (d, Ar-CH), 117.5 (d, Ar-CH), 114.6 (s, Ar-C), 114.4 (d, Ar-CH), 109.5 (d, Ar-CH), 107.3 (d, Ar-CH), 84.8 (d, Ar-CHCH
CH-Ar), 128.7 (d, 2C, Ar-CH), 128.6 (d, 2C, Ar-CH), 128.1 (s, Ar-C), 128.0 (d, 2C, Ar-CH), 127.4 (d, 2C, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.9 (d, Ar-CH), 117.5 (d, Ar-CH), 114.6 (s, Ar-C), 114.4 (d, Ar-CH), 109.5 (d, Ar-CH), 107.3 (d, Ar-CH), 84.8 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.7 (t, Ar-CH2OCHCH
CH), 72.7 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 71.1 (t, PhCH2O), 70.3 (t, PhCH2O), 56.1 (s, 3H, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C31H28BrO4]+ = [M + H]+: 543.1165; found 543.1142 and [C31H2881BrO4]+ = [M + H]+: 545.1145; found 545.1126, [C31H27BrNaO4]+ = [M + Na]+: 565.0985; found 565.0962 and [C31H2781BrNaO4]+ = [M + Na]+: 567.0964; found 567.0987.
CH), 71.1 (t, PhCH2O), 70.3 (t, PhCH2O), 56.1 (s, 3H, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C31H28BrO4]+ = [M + H]+: 543.1165; found 543.1142 and [C31H2881BrO4]+ = [M + H]+: 545.1145; found 545.1126, [C31H27BrNaO4]+ = [M + Na]+: 565.0985; found 565.0962 and [C31H2781BrNaO4]+ = [M + Na]+: 567.0964; found 567.0987.
5-(Benzyloxy)-1-[(E)-2-(2-bromo-4,5-dimethoxyphenyl)vinyl]-1,3-dihydro-2-benzofuran (6bg). GP-2 was carried out and the product 6bg (108 mg, 47%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1b) = 0.70, Rf(2g) = 0.15 and Rf(6bg) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2851, 1600, 1503, 1462, 1439, 1259, 1162, 1027, 801, 737, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.42 (dd, 2H, J = 8.3 and 1.5 Hz, Ar-H), 7.38 (ddd, 2H, J = 8.3, 5.8 and 1.5 Hz, Ar-H), 7.33 (ddd, 1H, J = 8.3, 5.8 and 1.5 Hz, Ar-H), 7.11 (d, 1H, J = 8.3 Hz, Ar-H), 7.02 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1b) = 0.70, Rf(2g) = 0.15 and Rf(6bg) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2922, 2851, 1600, 1503, 1462, 1439, 1259, 1162, 1027, 801, 737, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.42 (dd, 2H, J = 8.3 and 1.5 Hz, Ar-H), 7.38 (ddd, 2H, J = 8.3, 5.8 and 1.5 Hz, Ar-H), 7.33 (ddd, 1H, J = 8.3, 5.8 and 1.5 Hz, Ar-H), 7.11 (d, 1H, J = 8.3 Hz, Ar-H), 7.02 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.01 (s, 1H, Ar-H), 7.00 (s, 1H, Ar-H), 5.91 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.86 (d, 1H, J = 2.0 Hz, Ar-H), 6.09 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 7.01 (s, 1H, Ar-H), 7.00 (s, 1H, Ar-H), 5.91 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.86 (d, 1H, J = 2.0 Hz, Ar-H), 6.09 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.18 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH
CH], 5.18 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH
CH), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.07 (s, 2H, PhCH2O), 3.86 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.1 (s, Ar-C), 149.4 (s, Ar-C), 148.5 (s, Ar-C), 140.9 (s, Ar-C), 136.8 (s, Ar-C), 133.1 (s, Ar-C), 130.4 (d, Ar-CH–CH
CH), 5.07 (s, 2H, PhCH2O), 3.86 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.1 (s, Ar-C), 149.4 (s, Ar-C), 148.5 (s, Ar-C), 140.9 (s, Ar-C), 136.8 (s, Ar-C), 133.1 (s, Ar-C), 130.4 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.1 (d, Ar-CH–CH
CH-Ar), 130.1 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.5 (d, 2C, Ar-CH), 128.3 (s, Ar-C), 128.0 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.9 (d, Ar-CH), 115.2 (d, Ar-CH), 114.6 (d, Ar-CH), 114.5 (s, Ar-C), 109.1 (d, Ar-CH), 107.3 (d, Ar-CH), 84.9 (d, Ar-CHCH
CH-Ar), 128.5 (d, 2C, Ar-CH), 128.3 (s, Ar-C), 128.0 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.9 (d, Ar-CH), 115.2 (d, Ar-CH), 114.6 (d, Ar-CH), 114.5 (s, Ar-C), 109.1 (d, Ar-CH), 107.3 (d, Ar-CH), 84.9 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.7 (t, Ar-CH2OCHCH
CH), 72.7 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 70.3 (t, PhCH2O), 56.1 (q, Ar-OCH3), 55.9 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C25H24BrO4]+ = [M + H]+: 467.0852; found 467.0824 and [C25H2481BrO4]+ = [M + H]+: 469.0832; found 469.0817, [C25H23BrNaO4]+ = [M + Na]+: 489.0672; found 489.0646 and [C25H2381BrNaO4]+ = [M + Na]+: 491.0651; found 491.0649.
CH), 70.3 (t, PhCH2O), 56.1 (q, Ar-OCH3), 55.9 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C25H24BrO4]+ = [M + H]+: 467.0852; found 467.0824 and [C25H2481BrO4]+ = [M + H]+: 469.0832; found 469.0817, [C25H23BrNaO4]+ = [M + Na]+: 489.0672; found 489.0646 and [C25H2381BrNaO4]+ = [M + Na]+: 491.0651; found 491.0649.
1-[(E)-2-(2-Bromophenyl)vinyl]-5-methoxy-1,3-dihydro-2-benzo furan (6ca). GP-2 was carried out and the product 6ca (79 mg, 48%) was furnished as a yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1c) = 0.70, Rf(2a) = 0.40 and Rf(6ca) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2956, 2924, 2854, 1610, 1493, 1466, 1275, 1117, 1025, 821, 748, 665 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.54 (d, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.51 (d, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.22 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.15–7.00 (m, 3H, Ar-H and ArCH
10, Rf(1c) = 0.70, Rf(2a) = 0.40 and Rf(6ca) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2956, 2924, 2854, 1610, 1493, 1466, 1275, 1117, 1025, 821, 748, 665 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.54 (d, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.51 (d, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.22 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.15–7.00 (m, 3H, Ar-H and ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.83 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.79 (d, 1H, J = 2.4 Hz, Ar-H), 6.19 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.83 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.79 (d, 1H, J = 2.4 Hz, Ar-H), 6.19 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.18 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH
CH], 5.18 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH
CH), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 140.9 (s, Ar-C), 136.4 (s, Ar-C), 132.9 (d, Ar-CH), 132.7 (s, Ar-C), 132.4 (d, Ar-CH), 130.3 (d, Ar-CH–CH
CH), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 140.9 (s, Ar-C), 136.4 (s, Ar-C), 132.9 (d, Ar-CH), 132.7 (s, Ar-C), 132.4 (d, Ar-CH), 130.3 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 129.0 (d, Ar-CH–CH
CH-Ar), 129.0 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 127.4 (d, Ar-CH), 127.3 (d, Ar-CH), 123.8 (s, Ar-C), 122.8 (d, Ar-CH), 113.7 (d, Ar-CH), 106.3 (d, Ar-CH), 84.7 (d, Ar-CHCH
CH-Ar), 127.4 (d, Ar-CH), 127.3 (d, Ar-CH), 123.8 (s, Ar-C), 122.8 (d, Ar-CH), 113.7 (d, Ar-CH), 106.3 (d, Ar-CH), 84.7 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ar-CH2OCHCH
CH), 72.8 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 55.6 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H15BrNaO2]+ = [M + Na]: 353.0148; found 353.0164.
CH), 55.6 (q, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C17H15BrNaO2]+ = [M + Na]: 353.0148; found 353.0164.
1-[(E)-2-(2-Bromo-5-methoxyphenyl)vinyl]-5-methoxy-1,3-di hydro-2-benzofuran (6cc). GP-2 was carried out and the product 6cc (71 mg, 45%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1c) = 0.70, Rf(2c) = 0.50 and Rf(6cc) = 0.60 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2957, 2922, 2852, 1594, 1465, 1284, 1241, 1016, 804 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.42 (d, 1H, J = 8.8 Hz, Ar-H), δ = 7.10 (d, 1H, J = 8.3 Hz, Ar-H), δ = 7.04 (d, 1H, J = 3.3 Hz, Ar-H), 7.02 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1c) = 0.70, Rf(2c) = 0.50 and Rf(6cc) = 0.60 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2957, 2922, 2852, 1594, 1465, 1284, 1241, 1016, 804 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.42 (d, 1H, J = 8.8 Hz, Ar-H), δ = 7.10 (d, 1H, J = 8.3 Hz, Ar-H), δ = 7.04 (d, 1H, J = 3.3 Hz, Ar-H), 7.02 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.83 (dd, 1H, J = 8.3 and 1.9 Hz, Ar-H), 6.79 (d, 1H, J = 1.9 Hz, Ar-H), 6.69 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 6.18 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.83 (dd, 1H, J = 8.3 and 1.9 Hz, Ar-H), 6.79 (d, 1H, J = 1.9 Hz, Ar-H), 6.69 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 6.18 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.19 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH
CH], 5.19 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.10 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH
CH), 5.10 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.81 (s, 3H, Ar-OCH3), 3.76 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 158.9 (s, Ar-C), 140.9 (s, Ar-C), 137.1 (s, Ar-C), 133.5 (d, Ar-CH), 132.6 (s, Ar-C), 132.4 (d, Ar-CH–CH
CH), 3.81 (s, 3H, Ar-OCH3), 3.76 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 158.9 (s, Ar-C), 140.9 (s, Ar-C), 137.1 (s, Ar-C), 133.5 (d, Ar-CH), 132.6 (s, Ar-C), 132.4 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.5 (d, Ar-CH–CH
CH-Ar), 130.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 122.8 (d, Ar-CH), 115.7 (d, Ar-CH), 114.6 (s, Ar-C), 113.7 (d, Ar-CH), 112.0 (d, Ar-CH), 106.3 (d, Ar-CH), 84.7 (d, Ar-CHCH
CH-Ar), 122.8 (d, Ar-CH), 115.7 (d, Ar-CH), 114.6 (s, Ar-C), 113.7 (d, Ar-CH), 112.0 (d, Ar-CH), 106.3 (d, Ar-CH), 84.7 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ar-CH2OCHCH
CH), 72.8 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 55.6 (s, Ar-OCH3), 55.5 (s, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H16BrO2]+ = [M + Na]: 343.0328; found 343.0314.
CH), 55.6 (s, Ar-OCH3), 55.5 (s, Ar-OCH3) ppm. HR-MS (ESI+) m/z calculated for [C18H16BrO2]+ = [M + Na]: 343.0328; found 343.0314.
1-{(E)-2-[5-(Benzyloxy)-2-bromo-4-methoxyphenyl]vinyl}-5-methoxy-1,3-dihydro-2-benzofuran (6cd). GP-2 was carried out and the product 6cd (103 mg, 44%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1c) = 0.70, Rf(2d) = 0.30 and Rf(6cd) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2853, 1597, 1497, 1465, 1261, 1201, 1166, 1117, 1029, 813, 743, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.42 (d, 2H, J = 7.3 Hz, Ar-H), 7.37 (dd, 2H, J = 7.8 and 7.3 Hz, Ar-H), 7.31 (t, 1H, J = 7.3 Hz, Ar-H), 7.10 (d, 1H, J = 8.3 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.04 (s, 1H, Ar-H), 6.99 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1c) = 0.70, Rf(2d) = 0.30 and Rf(6cd) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2853, 1597, 1497, 1465, 1261, 1201, 1166, 1117, 1029, 813, 743, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.42 (d, 2H, J = 7.3 Hz, Ar-H), 7.37 (dd, 2H, J = 7.8 and 7.3 Hz, Ar-H), 7.31 (t, 1H, J = 7.3 Hz, Ar-H), 7.10 (d, 1H, J = 8.3 Hz, Ar-H), 7.06 (s, 1H, Ar-H), 7.04 (s, 1H, Ar-H), 6.99 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.84 (dd, 1H, J = 8.3 and 2.0 Hz, Ar-H), 6.79 (d, 1H, J = 2.0 Hz, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.84 (dd, 1H, J = 8.3 and 2.0 Hz, Ar-H), 6.79 (d, 1H, J = 2.0 Hz, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.75 [d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.19 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH
CH], 5.19 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.10 (s, 2H, PhCH2O), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH
CH), 5.10 (s, 2H, PhCH2O), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.83 (s, 3H, Ar-OCH3), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.9 (s, Ar-C), 149.1 (s, Ar-C), 148.6 (s, Ar-C), 140.9 (s, Ar-C), 136.2 (s, Ar-C), 132.7 (s, Ar-C), 130.4 (d, Ar-CH–CH
CH), 3.83 (s, 3H, Ar-OCH3), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 159.9 (s, Ar-C), 149.1 (s, Ar-C), 148.6 (s, Ar-C), 140.9 (s, Ar-C), 136.2 (s, Ar-C), 132.7 (s, Ar-C), 130.4 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.3 (d, Ar-CH–CH
CH-Ar), 130.3 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.8 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.9 (d, Ar-CH), 117.5 (d, Ar-CH), 114.3 (s, Ar-C), 113.7 (d, Ar-CH), 109.5 (d, Ar-CH), 106.2 (d, Ar-CH), 84.9 (d, Ar-CHCH
CH-Ar), 128.8 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 122.9 (d, Ar-CH), 117.5 (d, Ar-CH), 114.3 (s, Ar-C), 113.7 (d, Ar-CH), 109.5 (d, Ar-CH), 106.2 (d, Ar-CH), 84.9 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.7 (t, Ar-CH2OCHCH
CH), 72.7 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 71.1 (t, PhCH2O), 56.1 (q, Ar-OCH3), 55.5 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C25H24BrO4]+ = [M + H]+: 467.0852; found 467.0826 and [C25H2481BrO4]+ = [M + H]+: 469.0832; found 469.0812.
CH), 71.1 (t, PhCH2O), 56.1 (q, Ar-OCH3), 55.5 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C25H24BrO4]+ = [M + H]+: 467.0852; found 467.0826 and [C25H2481BrO4]+ = [M + H]+: 469.0832; found 469.0812.
5-Bromo-6-[(E)-2-(5-methoxy-1,3-dihydro-2-benzofuran-1-yl) vinyl]-1,3-benzodioxole (6cf). GP-2 was carried out and the product 6cf (80 mg, 43%) was furnished as a pale yellow liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1c) = 0.70, Rf(2f) = 0.50 and Rf(6cf) = 0.55 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2852, 1605, 1500, 1474, 1235, 1106, 1036, 932, 870, 822 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.07 (d, 1H, J = 8.3 Hz, Ar-H), 6.99 (s, 2H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1c) = 0.70, Rf(2f) = 0.50 and Rf(6cf) = 0.55 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2921, 2852, 1605, 1500, 1474, 1235, 1106, 1036, 932, 870, 822 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.07 (d, 1H, J = 8.3 Hz, Ar-H), 6.99 (s, 2H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.82 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.77 (d, 1H, J = 2.4 Hz, Ar-H), 6.04 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.82 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.77 (d, 1H, J = 2.4 Hz, Ar-H), 6.04 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.94 (s, 2H, OCH2O), 5.72 [d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.94 (s, 2H, OCH2O), 5.72 [d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.17 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH
CH], 5.17 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.08 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH
CH), 5.08 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 148.1 (s, Ar-C), 147.6 (s, Ar-C), 140.8 (s, Ar-C), 132.7 (s, Ar-C), 130.6 (d, Ar-CH–CH
CH), 3.81 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 148.1 (s, Ar-C), 147.6 (s, Ar-C), 140.8 (s, Ar-C), 132.7 (s, Ar-C), 130.6 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.2 (d, Ar-CH–CH
CH-Ar), 130.2 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 129.7 (s, Ar-C), 122.7 (d, Ar-CH), 115.0 (s, Ar-C), 113.7 (d, Ar-CH), 112.6 (d, Ar-CH), 106.4 (d, Ar-CH), 106.2 (d, Ar-CH), 101.7 (t, OCH2O), 84.7 (d, Ar-CHCH
CH-Ar), 129.7 (s, Ar-C), 122.7 (d, Ar-CH), 115.0 (s, Ar-C), 113.7 (d, Ar-CH), 112.6 (d, Ar-CH), 106.4 (d, Ar-CH), 106.2 (d, Ar-CH), 101.7 (t, OCH2O), 84.7 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.7 (t, Ar-CH2OCHCH
CH), 72.7 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 55.5 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C18H16BrO4]+ = [M + H]+: 375.0226; found 375.0212 and [C18H1681BrO4]+ = [M + H]+: 377.0206; found 377.0189.
CH), 55.5 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C18H16BrO4]+ = [M + H]+: 375.0226; found 375.0212 and [C18H1681BrO4]+ = [M + H]+: 377.0206; found 377.0189.
1-[(E)-2-(2-Bromo-3,4,5-trimethoxyphenyl)vinyl]-5-methoxy-1,3-dihydro-2-benzofuran (6ch). GP-2 was carried out and the product 6ch (86 mg, 41%) was furnished as a pale yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, Rf(1c) = 0.95, Rf(2h) = 0.25 and Rf(6ch) = 0.45 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1563, 1481, 1463, 1427, 1392, 1326, 1274, 1200, 1165, 1107, 1031, 1011, 926, 813 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.08 (d, 1H, J = 8.8 Hz, Ar-H), 7.06 (d, 1H, J = 15.6 Hz, ArCH
20, Rf(1c) = 0.95, Rf(2h) = 0.25 and Rf(6ch) = 0.45 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2923, 2852, 1563, 1481, 1463, 1427, 1392, 1326, 1274, 1200, 1165, 1107, 1031, 1011, 926, 813 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.08 (d, 1H, J = 8.8 Hz, Ar-H), 7.06 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.86 (s, 1H, Ar-H), 6.83 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.78 (d, 1H, J = 2.4 Hz, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.86 (s, 1H, Ar-H), 6.83 (dd, 1H, J = 8.3 and 2.4 Hz, Ar-H), 6.78 (d, 1H, J = 2.4 Hz, Ar-H), 6.10 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.75 (d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.75 (d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.18 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH
CH), 5.18 (dd, 1H, J = 12.2 and 2.4 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH
CH), 5.09 (d, 1H, J = 12.2 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.88 (s, 3H, Ar-OCH3), 3.87 (s, 3H, Ar-OCH3), 3.82 (s, 3H, Ar-OCH3), 3.80 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 152.6 (s, Ar-C), 150.8 (s, Ar-C), 143.0 (s, Ar-C), 140.8 (s, Ar-C), 132.6 (s, Ar-C), 131.9 (s, Ar-C), 131.5 (d, Ar-CH–CH
CH), 3.88 (s, 3H, Ar-OCH3), 3.87 (s, 3H, Ar-OCH3), 3.82 (s, 3H, Ar-OCH3), 3.80 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 160.0 (s, Ar-C), 152.6 (s, Ar-C), 150.8 (s, Ar-C), 143.0 (s, Ar-C), 140.8 (s, Ar-C), 132.6 (s, Ar-C), 131.9 (s, Ar-C), 131.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.6 (d, Ar-CH–CH
CH-Ar), 130.6 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 122.8 (d, Ar-CH), 113.7 (d, Ar-CH), 110.8 (s, Ar-C), 106.2 (d, Ar-CH), 105.6 (d, Ar-CH), 84.7 (d, Ar-CHCH
CH-Ar), 122.8 (d, Ar-CH), 113.7 (d, Ar-CH), 110.8 (s, Ar-C), 106.2 (d, Ar-CH), 105.6 (d, Ar-CH), 84.7 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.7 (t, Ar-CH2OCHCH
CH), 72.7 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 61.1 (q, Ar-OCH3), 60.9 (q, Ar-OCH3), 56.1 (q, Ar-OCH3), 55.5 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C20H21BrNaO5]+ = [M + Na]+: 443.0465; found 443.0448.
CH), 61.1 (q, Ar-OCH3), 60.9 (q, Ar-OCH3), 56.1 (q, Ar-OCH3), 55.5 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C20H21BrNaO5]+ = [M + Na]+: 443.0465; found 443.0448.
5-{(E)-2-[5-(Benzyloxy)-2-bromophenyl]vinyl}-5,7-dihydrofuro [3,4-f][1,3]benzodioxole (6fb). GP-2 was carried out and the product 6fb (92 mg, 41%) was furnished as pale yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, Rf(1f) = 0.30, Rf(2b) = 0.45 and Rf(6fb) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2920, 2851, 1591, 1501, 1464, 1378, 1278, 1239, 1173, 1122, 1039, 939, 851, 737, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.50–7.27 (m, 6H, Ar-H), 7.14 (d, 1H, J = 2.9 Hz, Ar-H), 7.01 (d, 1H, J = 15.6 Hz, ArCH
10, Rf(1f) = 0.30, Rf(2b) = 0.45 and Rf(6fb) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2920, 2851, 1591, 1501, 1464, 1378, 1278, 1239, 1173, 1122, 1039, 939, 851, 737, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.50–7.27 (m, 6H, Ar-H), 7.14 (d, 1H, J = 2.9 Hz, Ar-H), 7.01 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.76 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 6.68 (s, 1H, Ar-H), 6.62 (s, 1H, Ar-H), 6.14 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.76 (dd, 1H, J = 8.8 and 2.9 Hz, Ar-H), 6.68 (s, 1H, Ar-H), 6.62 (s, 1H, Ar-H), 6.14 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.97 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.97 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.70 (d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.97 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.97 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.70 (d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.12 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.12 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.04 (d, 1H, J = 11.7 Hz, ArCHaHbOCHCH
CH), 5.04 (d, 1H, J = 11.7 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.01 (s, 2H, PhCH2O) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 158.1 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 137.1 (s, Ar-C), 136.4 (s, Ar-C), 133.5 (d, Ar-CH–CH
CH), 5.01 (s, 2H, PhCH2O) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 158.1 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 137.1 (s, Ar-C), 136.4 (s, Ar-C), 133.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 133.4 (s, Ar-C), 132.3 (d, Ar-CH–CH
CH-Ar), 133.4 (s, Ar-C), 132.3 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 131.9 (s, Ar-C), 130.5 (d, Ar-CH), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 116.3 (d, Ar-CH), 114.8 (s, Ar-C), 113.3 (d, Ar-CH), 102.6 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 84.9 (d, Ar-CHCH
CH-Ar), 131.9 (s, Ar-C), 130.5 (d, Ar-CH), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.4 (d, 2C, Ar-CH), 116.3 (d, Ar-CH), 114.8 (s, Ar-C), 113.3 (d, Ar-CH), 102.6 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 84.9 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.9 (t, Ar-CH2OCHCH
CH), 72.9 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 70.2 (t, PhCH2O) ppm. HR-MS (ESI+): m/z calculated for [C24H19BrNaO4]+ = [M + Na]+: 473.0359; found 473.0330 and [C24H1981BrNaO4]+ = [M + Na]+: 475.0338; found 475.0317.
CH), 70.2 (t, PhCH2O) ppm. HR-MS (ESI+): m/z calculated for [C24H19BrNaO4]+ = [M + Na]+: 473.0359; found 473.0330 and [C24H1981BrNaO4]+ = [M + Na]+: 475.0338; found 475.0317.
5-{(E)-2-[4-(Benzyloxy)-2-bromo-5-methoxyphenyl]vinyl}-5,7-dihydrofuro[3,4-f][1,3]benzodioxole (6fe). GP-2 was carried out and the product 6fe (113 mg, 47%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, Rf(1f) = 0.70, Rf(2e) = 0.30 and Rf(6fe) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2956, 2924, 2853, 1598, 1502, 1439, 1259, 1162, 1033, 852, 803, 735, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.41 (d, 2H, J = 7.3 Hz, Ar-H), 7.34 (dd, 2H, J = 7.8 and 7.3 Hz, Ar-H), 7.30 (t, 1H, J = 7.3 Hz, Ar-H), 7.07 (s, 1H, Ar-H), 7.02 (s, 1H, Ar-H), 6.95 (d, 1H, J = 15.6 Hz, ArCH
20, Rf(1f) = 0.70, Rf(2e) = 0.30 and Rf(6fe) = 0.50 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2956, 2924, 2853, 1598, 1502, 1439, 1259, 1162, 1033, 852, 803, 735, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.41 (d, 2H, J = 7.3 Hz, Ar-H), 7.34 (dd, 2H, J = 7.8 and 7.3 Hz, Ar-H), 7.30 (t, 1H, J = 7.3 Hz, Ar-H), 7.07 (s, 1H, Ar-H), 7.02 (s, 1H, Ar-H), 6.95 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.68 (s, 1H, Ar-H), 6.60 (s, 1H, Ar-H), 6.96 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.68 (s, 1H, Ar-H), 6.60 (s, 1H, Ar-H), 6.96 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.97 (s, 2H, OCH2O), 5.67 (d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.97 (s, 2H, OCH2O), 5.67 (d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.11 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.11 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.07 (s, 2H, PhCH2O), 5.02 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.07 (s, 2H, PhCH2O), 5.02 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.85 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 150.2 (s, Ar-C), 148.0 (s, Ar-C), 147.6 (s, 2C, Ar-C), 136.5 (s, Ar-C), 133.6 (s, Ar-C), 131.9 (s, Ar-C), 130.5 (d, Ar-CH–CH
CH), 3.85 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 150.2 (s, Ar-C), 148.0 (s, Ar-C), 147.6 (s, 2C, Ar-C), 136.5 (s, Ar-C), 133.6 (s, Ar-C), 131.9 (s, Ar-C), 130.5 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.0 (d, Ar-CH–CH
CH-Ar), 130.0 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.5 (d, 2C, Ar-CH), 128.3 (s, Ar-C), 128.0 (d, Ar-CH), 127.5 (d, 2C, Ar-CH), 115.7 (d, Ar-CH), 115.1 (s, Ar-C), 112.1 (d, Ar-CH), 102.6 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 85.1 (d, Ar-CHCH
CH-Ar), 128.5 (d, 2C, Ar-CH), 128.3 (s, Ar-C), 128.0 (d, Ar-CH), 127.5 (d, 2C, Ar-CH), 115.7 (d, Ar-CH), 115.1 (s, Ar-C), 112.1 (d, Ar-CH), 102.6 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 85.1 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ar-CH2OCHCH
CH), 72.8 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 71.2 (t, PhCH2O), 56.2 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C25H22BrO5]+ = [M + H]+: 481.0645; found 481.0615 and [C25H2281BrO5]+ = [M + H]+: 483.0625; found 483.0602, [C25H21BrNaO5]+ = [M + Na]+: 503.0465; found 503.0438 and [C25H21Br81NaO5]+ = [M + Na]+: 505.0444; found 505.0422.
CH), 71.2 (t, PhCH2O), 56.2 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C25H22BrO5]+ = [M + H]+: 481.0645; found 481.0615 and [C25H2281BrO5]+ = [M + H]+: 483.0625; found 483.0602, [C25H21BrNaO5]+ = [M + Na]+: 503.0465; found 503.0438 and [C25H21Br81NaO5]+ = [M + Na]+: 505.0444; found 505.0422.
5-[(E)-2-(2-Bromo-4,5-dimethoxyphenyl)vinyl]-5,7-dihydrofuro [3,4-f][1,3]benzodioxole (6fg). GP-2 was carried out and the product 6fg (85 mg, 42%) was furnished as a pale yellow viscous liquid. [TLC control (petroleum ether/ethyl acetate 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, Rf(1f) = 0.70, Rf(2g) = 0.30 and Rf(6fg) = 0.55 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2852, 1600, 1503, 1473, 1380, 1261, 1208, 1163, 1035, 937, 860, 736, 698, 665 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.00 (s, 2H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH
20, Rf(1f) = 0.70, Rf(2g) = 0.30 and Rf(6fg) = 0.55 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2852, 1600, 1503, 1473, 1380, 1261, 1208, 1163, 1035, 937, 860, 736, 698, 665 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.00 (s, 2H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.67 (s, 1H, Ar-H), 6.63 (s, 1H, Ar-H), 6.06 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.67 (s, 1H, Ar-H), 6.63 (s, 1H, Ar-H), 6.06 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.97 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.96 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.69 (d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.97 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.96 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.69 (d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.11 (dd, 1H, J = 11.7 and 2.0 Hz, ArCHaHbOCHCH
CH), 5.11 (dd, 1H, J = 11.7 and 2.0 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.02 (dd, 1H, J = 11.7 and 2.0 Hz, ArCHaHbOCHCH
CH), 5.02 (dd, 1H, J = 11.7 and 2.0 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.86 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.5 (s, Ar-C), 148.5 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 133.6 (s, Ar-C), 131.9 (s, Ar-C), 130.7 (d, Ar-CH–CH
CH), 3.86 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.5 (s, Ar-C), 148.5 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 133.6 (s, Ar-C), 131.9 (s, Ar-C), 130.7 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.0 (d, Ar-CH–CH
CH-Ar), 130.0 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.2 (s, Ar-C), 115.3 (d, Ar-CH), 114.6 (s, Ar-C), 109.1 (d, Ar-CH), 102.7 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 85.2 (d, Ar-CHCH
CH-Ar), 128.2 (s, Ar-C), 115.3 (d, Ar-CH), 114.6 (s, Ar-C), 109.1 (d, Ar-CH), 102.7 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 85.2 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ar-CH2OCHCH
CH), 72.8 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 56.1 (q, Ar-OCH3), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C19H17BrNaO5]+ = [M + Na]+: 427.0152; found 427.0127 and [C19H1781BrNaO5]+ = [M + Na]+: 429.0137; found 429.0121, HR-MS (ESI+) m/z calculated for [C19H18BrO5]+ = [M + H]+: 405.0332; found 405.0304 and [C19H1881BrO5]+ = [M + H]+: 407.0312; found 407.0294.
CH), 56.1 (q, Ar-OCH3), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C19H17BrNaO5]+ = [M + Na]+: 427.0152; found 427.0127 and [C19H1781BrNaO5]+ = [M + Na]+: 429.0137; found 429.0121, HR-MS (ESI+) m/z calculated for [C19H18BrO5]+ = [M + H]+: 405.0332; found 405.0304 and [C19H1881BrO5]+ = [M + H]+: 407.0312; found 407.0294.
5-[(E)-2-(2-Bromo-3,4,5-trimethoxyphenyl)vinyl]-5,7-dihydro furo[3,4-f][1,3]benzodioxole (6fh). GP-2 was carried out and the product 6fh (98 mg, 45%) was furnished as a pale brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, Rf(1f) = 0.70, Rf(2h) = 0.20 and Rf(6fh) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2928, 2854, 1566, 1503, 1482, 1394, 1329, 1264, 1198, 1164, 1107, 1037, 1010, 934, 814, 739 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.05 (d, 1H, J = 15.6 Hz, ArCH
20, Rf(1f) = 0.70, Rf(2h) = 0.20 and Rf(6fh) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2928, 2854, 1566, 1503, 1482, 1394, 1329, 1264, 1198, 1164, 1107, 1037, 1010, 934, 814, 739 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.05 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.86 (s, 1H, Ar-H), 6.68 (s, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 6.07 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.86 (s, 1H, Ar-H), 6.68 (s, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 6.07 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.96 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.95 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.69 (d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.96 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.95 (d, 1H, J = 2.9 Hz, OCHaHbO), 5.69 (d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.11 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.11 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.03 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.03 (dd, 1H, J = 11.7 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.88 (s, 3H, Ar-OCH3), 3.87 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 152.6 (s, Ar-C), 150.8 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 143.0 (s, Ar-C), 133.4 (s, Ar-C), 131.9 (s, Ar-C), 131.8 (s, Ar-C), 131.3 (d, Ar-CH–CH
CH), 3.88 (s, 3H, Ar-OCH3), 3.87 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 152.6 (s, Ar-C), 150.8 (s, Ar-C), 148.0 (s, Ar-C), 147.7 (s, Ar-C), 143.0 (s, Ar-C), 133.4 (s, Ar-C), 131.9 (s, Ar-C), 131.8 (s, Ar-C), 131.3 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.8 (d, Ar-CH–CH
CH-Ar), 130.8 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 110.8 (s, Ar-C), 105.6 (d, Ar-CH), 102.6 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 85.0 (d, Ar-CHCH
CH-Ar), 110.8 (s, Ar-C), 105.6 (d, Ar-CH), 102.6 (d, Ar-CH), 101.6 (d, Ar-CH), 101.5 (t, OCH2O), 85.0 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.9 (t, Ar-CH2OCHCH
CH), 72.9 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 61.1 (q, Ar-OCH3), 60.9 (q, Ar-OCH3), 56.1 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C20H19BrNaO6]+ = [M + Na]+: 457.0257; found 457.0257 and [C20H1981BrNaO6]+ = [M + Na]+: 459.0237; found 459.0236.
CH), 61.1 (q, Ar-OCH3), 60.9 (q, Ar-OCH3), 56.1 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C20H19BrNaO6]+ = [M + Na]+: 457.0257; found 457.0257 and [C20H1981BrNaO6]+ = [M + Na]+: 459.0237; found 459.0236.
1-{(E)-2-[5-(Benzyloxy)-2-bromo-4-methoxyphenyl]vinyl}-5,6-dimethoxy-1,3-dihydro-2-benzofuran (6gd). GP-2 was carried out and the product 6gd (116 mg, 47%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, Rf(1g) = 0.65, Rf(2d) = 0.55 and Rf(6gd) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2926, 2853, 1598, 1503, 1463, 1384, 1261, 1203, 1166, 1032, 859, 737, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.41 (d, 2H, J = 7.3 Hz, Ar-H), 7.36 (dd, 2H, J = 7.8 and 7.3 Hz, Ar-H), 7.31 (t, 1H, J = 7.3 Hz, Ar-H), 7.05 (s, 2H, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH
30, Rf(1g) = 0.65, Rf(2d) = 0.55 and Rf(6gd) = 0.40 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2926, 2853, 1598, 1503, 1463, 1384, 1261, 1203, 1166, 1032, 859, 737, 698 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.41 (d, 2H, J = 7.3 Hz, Ar-H), 7.36 (dd, 2H, J = 7.8 and 7.3 Hz, Ar-H), 7.31 (t, 1H, J = 7.3 Hz, Ar-H), 7.05 (s, 2H, Ar-H), 7.00 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.77 (s, 1H, Ar-H), 6.69 (s, 1H, Ar-H), 6.10 (dd, 1H, J = 15.6 and 8.3 Hz, ArCH
CH), 6.77 (s, 1H, Ar-H), 6.69 (s, 1H, Ar-H), 6.10 (dd, 1H, J = 15.6 and 8.3 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.75 (d, 1H, J = 8.3 Hz, ArCH(O)CH
CH), 5.75 (d, 1H, J = 8.3 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.18 (dd, 1H, J = 11.7 and 2.0 Hz, ArCHaHbOCHCH
CH), 5.18 (dd, 1H, J = 11.7 and 2.0 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.10 (s, 2H, PhCH2O), 5.07 (dd, 1H, J = 11.2 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.10 (s, 2H, PhCH2O), 5.07 (dd, 1H, J = 11.2 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.88 (s, 3H, Ar-OCH3), 3.86 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.3 (s, Ar-C), 149.1 (s, Ar-C), 149.0 (s, Ar-C), 148.6 (s, Ar-C), 136.2 (s, Ar-C), 132.1 (s, Ar-C), 130.6 (s, Ar-C), 130.6 (d, Ar-CH–CH
CH), 3.88 (s, 3H, Ar-OCH3), 3.86 (s, 3H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.3 (s, Ar-C), 149.1 (s, Ar-C), 149.0 (s, Ar-C), 148.6 (s, Ar-C), 136.2 (s, Ar-C), 132.1 (s, Ar-C), 130.6 (s, Ar-C), 130.6 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.2 (d, Ar-CH–CH
CH-Ar), 130.2 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.7 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.3 (d, 2C, Ar-CH), 117.5 (d, Ar-CH), 114.4 (s, Ar-C), 109.5 (d, Ar-CH), 104.9 (d, Ar-CH), 103.9 (d, Ar-CH), 85.6 (d, Ar-CHCH
CH-Ar), 128.7 (s, Ar-C), 128.6 (d, 2C, Ar-CH), 128.1 (d, Ar-CH), 127.3 (d, 2C, Ar-CH), 117.5 (d, Ar-CH), 114.4 (s, Ar-C), 109.5 (d, Ar-CH), 104.9 (d, Ar-CH), 103.9 (d, Ar-CH), 85.6 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 73.0 (t, Ar-CH2OCHCH
CH), 73.0 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 71.1 (t, PhCH2O), 56.2 (q, Ar-OCH3), 56.1 (q, Ar-OCH3), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C26H25BrNaO5]+ = [M + Na]+: 519.0778; found 519.0753 and [C26H2581BrNaO5]+ = [M + Na]+: 521.0757; found 521.0735.
CH), 71.1 (t, PhCH2O), 56.2 (q, Ar-OCH3), 56.1 (q, Ar-OCH3), 56.0 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C26H25BrNaO5]+ = [M + Na]+: 519.0778; found 519.0753 and [C26H2581BrNaO5]+ = [M + Na]+: 521.0757; found 521.0735.
1-[(E)-2-(2-Bromo-4,5-dimethoxyphenyl)vinyl]-5,6-dimethoxy-1,3-dihydro-2-benzofuran (6gg). GP-2 was carried out and the product 6gg (90 mg, 43%) was furnished as a brown viscous liquid. [TLC control (petroleum ether/ethyl acetate 70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, Rf(1g) = 0.65, Rf(2g) = 0.45 and Rf(6gg) = 0.35 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2852, 1600, 1504, 1462, 1264, 1210, 1163, 1121, 1029, 863 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.02 (s, 1H, Ar-H), 7.01 (d, 1H, J = 15.6 Hz, ArCH
30, Rf(1g) = 0.65, Rf(2g) = 0.45 and Rf(6gg) = 0.35 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2852, 1600, 1504, 1462, 1264, 1210, 1163, 1121, 1029, 863 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.02 (s, 1H, Ar-H), 7.01 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.00 (s, 1H, Ar-H), 6.77 (s, 1H, Ar-H), 6.69 (s, 1H, Ar-H), 6.09 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 7.00 (s, 1H, Ar-H), 6.77 (s, 1H, Ar-H), 6.69 (s, 1H, Ar-H), 6.09 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.74 (d, 1H, J = 7.8 Hz, ArCH(O)CH
CH), 5.74 (d, 1H, J = 7.8 Hz, ArCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.17 (dd, 1H, J = 11.2 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.17 (dd, 1H, J = 11.2 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.07 (dd, 1H, J = 11.2 and 2.9 Hz, ArCHaHbOCHCH
CH), 5.07 (dd, 1H, J = 11.2 and 2.9 Hz, ArCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.88 (s, 3H, Ar-OCH3), 3.86 (s, 6H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.5 (s, Ar-C), 149.4 (s, Ar-C), 149.1 (s, Ar-C), 148.5 (s, Ar-C), 132.2 (s, Ar-C), 130.7 (s, Ar-C), 130.6 (d, Ar-CH–CH
CH), 3.88 (s, 3H, Ar-OCH3), 3.86 (s, 6H, Ar-OCH3), 3.83 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 149.5 (s, Ar-C), 149.4 (s, Ar-C), 149.1 (s, Ar-C), 148.5 (s, Ar-C), 132.2 (s, Ar-C), 130.7 (s, Ar-C), 130.6 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.1 (d, Ar-CH–CH
CH-Ar), 130.1 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.3 (s, Ar-C), 115.3 (d, Ar-CH), 114.6 (s, Ar-C), 109.1 (d, Ar-CH), 105.0 (d, Ar-CH), 104.0 (d, Ar-CH), 85.6 (d, Ar-CHCH
CH-Ar), 128.3 (s, Ar-C), 115.3 (d, Ar-CH), 114.6 (s, Ar-C), 109.1 (d, Ar-CH), 105.0 (d, Ar-CH), 104.0 (d, Ar-CH), 85.6 (d, Ar-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 73.0 (t, Ar-CH2OCHCH
CH), 73.0 (t, Ar-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 56.2 (q, Ar-OCH3), 56.1 (q, Ar-OCH3), 56.0 (q, Ar-OCH3), 55.9 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C20H21BrNaO5]+ = [M + Na]+: 443.0465; found 443.0468.
CH), 56.2 (q, Ar-OCH3), 56.1 (q, Ar-OCH3), 56.0 (q, Ar-OCH3), 55.9 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C20H21BrNaO5]+ = [M + Na]+: 443.0465; found 443.0468.
(2E)-3-[2-(Hydroxymethyl)phenyl]-1-(2-methylphenyl)prop-2-en-1-ol (5ai). GP-3 was carried out and the product 5ai (65 mg, 97%) was furnished as a yellow colored viscous liquid. [TLC control (petroleum ether/ethyl acetate 70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, Rf(3ai) = 0.70, Rf(5ai) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3330, 1485, 1459, 1006, 967, 753, 564 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.52–7.46 (m, 1H, Ar-H), 7.44 (d, 1H, J = 7.8 Hz, Ar-H), 7.32–7.10 (m, 6H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH
30, Rf(3ai) = 0.70, Rf(5ai) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3330, 1485, 1459, 1006, 967, 753, 564 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.52–7.46 (m, 1H, Ar-H), 7.44 (d, 1H, J = 7.8 Hz, Ar-H), 7.32–7.10 (m, 6H, Ar-H), 6.98 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.21 (dd, 1H, J = 15.6 and 5.9 Hz, ArCH
CH), 6.21 (dd, 1H, J = 15.6 and 5.9 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.47 [d, 1H, J = 5.9 Hz, PhCH(OH)CH
CH), 5.47 [d, 1H, J = 5.9 Hz, PhCH(OH)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 4.63 (d, 1H, J = 12.2 Hz, PhCHaHbOH), 4.62 (d, 1H, J = 12.2 Hz, PhCHaHbOH), 3.76 (br.s, 1H, OH), 3.29 (br.s, 1H, OH), 2.35 (s, 3H, Ar-CH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 140.4 (s, Ar-C), 137.5 (s, Ar-C), 135.8 (s, Ar-C), 135.2 (s, Ar-C), 133.2 (d, Ar-CH–CH
CH], 4.63 (d, 1H, J = 12.2 Hz, PhCHaHbOH), 4.62 (d, 1H, J = 12.2 Hz, PhCHaHbOH), 3.76 (br.s, 1H, OH), 3.29 (br.s, 1H, OH), 2.35 (s, 3H, Ar-CH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 140.4 (s, Ar-C), 137.5 (s, Ar-C), 135.8 (s, Ar-C), 135.2 (s, Ar-C), 133.2 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.4 (d, Ar-CH), 128.7 (d, Ar-CH–CH
CH-Ar), 130.4 (d, Ar-CH), 128.7 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.1 (d, Ar-CH), 127.6 (d, Ar-CH), 127.5 (d, Ar-CH), 126.9 (d, Ar-CH), 126.2 (2 × d, 2C, Ar-CH), 125.9 (d, Ar-CH), 71.4 (d, Ph-CHCH
CH-Ar), 128.1 (d, Ar-CH), 127.6 (d, Ar-CH), 127.5 (d, Ar-CH), 126.9 (d, Ar-CH), 126.2 (2 × d, 2C, Ar-CH), 125.9 (d, Ar-CH), 71.4 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 63.1 (t, Ph-CH2OH), 19.1 (q, Ar-CH3) ppm. HR-MS (ESI+): m/z calculated for [C17H18NaO2]+ = [M + Na]+: 277.1199; found 277.1197.
CH), 63.1 (t, Ph-CH2OH), 19.1 (q, Ar-CH3) ppm. HR-MS (ESI+): m/z calculated for [C17H18NaO2]+ = [M + Na]+: 277.1199; found 277.1197.
(2E)-3-[2-(Hydroxymethyl)phenyl]-1-(2-methoxyphenyl)prop-2-en-1-ol (5aj). GP-3 was carried out and the product 5aj (67 mg, 96%) was furnished as a colorless viscous liquid. [TLC control (petroleum ether/ethyl acetate 70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, Rf(3aj) = 0.80, Rf(5aj) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3320, 1597, 1489, 1461, 1244, 1023, 753 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.39 (ddd, 2H, J = 8.8, 7.8 and 1.5 Hz, Ar-H), 7.32–7.10 (m, 4H, Ar-H), 6.99 (d, 1H, J = 16.1 Hz, ArCH
30, Rf(3aj) = 0.80, Rf(5aj) = 0.30 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 3320, 1597, 1489, 1461, 1244, 1023, 753 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.39 (ddd, 2H, J = 8.8, 7.8 and 1.5 Hz, Ar-H), 7.32–7.10 (m, 4H, Ar-H), 6.99 (d, 1H, J = 16.1 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.87 (dd, 1H, J = 7.8 and 7.3 Hz, Ar-H), 6.82 (d, 1H, J = 8.3 Hz, Ar-H), 6.43 (dd, 1H, J = 16.1 and 5.9 Hz, ArCH
CH), 6.87 (dd, 1H, J = 7.8 and 7.3 Hz, Ar-H), 6.82 (d, 1H, J = 8.3 Hz, Ar-H), 6.43 (dd, 1H, J = 16.1 and 5.9 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.52 [d, 1H, J = 5.9 Hz, PhCH(OH)CH
CH), 5.52 [d, 1H, J = 5.9 Hz, PhCH(OH)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 4.66 (s, 2H, ArCH2OH), 3.77 (s, 3H, Ar-OCH3), 3.64 (br.s, 2H, 2 × OH) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 156.7 (s, Ar-C), 141.1 (s, Ar-C), 138.5 (s, Ar-C), 131.0 (d, Ar-CH–CH
CH], 4.66 (s, 2H, ArCH2OH), 3.77 (s, 3H, Ar-OCH3), 3.64 (br.s, 2H, 2 × OH) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 156.7 (s, Ar-C), 141.1 (s, Ar-C), 138.5 (s, Ar-C), 131.0 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 130.0 (d, Ar-CH), 128.8 (d, Ar-CH–CH
CH-Ar), 130.0 (d, Ar-CH), 128.8 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.3 (d, Ar-CH), 128.1 (d, Ar-CH), 127.8 (d, Ar-CH), 127.0 (d, Ar-CH), 125.5 (s, Ar-C), 125.4 (d, Ar-CH), 120.6 (d, Ar-CH), 110.8 (d, Ar-CH), 73.2 (d, Ph-CHCH
CH-Ar), 128.3 (d, Ar-CH), 128.1 (d, Ar-CH), 127.8 (d, Ar-CH), 127.0 (d, Ar-CH), 125.5 (s, Ar-C), 125.4 (d, Ar-CH), 120.6 (d, Ar-CH), 110.8 (d, Ar-CH), 73.2 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 63.5 (t, Ph-CH2OH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C17H19O3]+ = [M + H]+: 271.1329; found 271.1320.
CH), 63.5 (t, Ph-CH2OH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C17H19O3]+ = [M + H]+: 271.1329; found 271.1320.
1-[(E)-2-(2-Methylphenyl)vinyl]-1,3-dihydro-2-benzofuran (6ai). GP-3 was carried out and the product 6ai (50 mg, 84%) was furnished as a colorless viscous liquid. [TLC control (petroleum ether/ethyl acetate 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, Rf(5ai) = 0.15, Rf(6ai) = 0.80 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2853, 1731, 1460, 1029, 965, 747, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.45 (d, 1H, J = 8.3 Hz, Ar-H), 7.36–7.25 (m, 3H, Ar-H), 7.24–7.10 (m, 4H, Ar-H), 6.97 (d, 1H, J = 15.6 Hz, ArCH
5, Rf(5ai) = 0.15, Rf(6ai) = 0.80 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2924, 2853, 1731, 1460, 1029, 965, 747, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.45 (d, 1H, J = 8.3 Hz, Ar-H), 7.36–7.25 (m, 3H, Ar-H), 7.24–7.10 (m, 4H, Ar-H), 6.97 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.16 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.16 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.79 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.79 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.14 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH
CH), 5.14 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.39 (s, 3H, Ar-CH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 141.0 (s, Ar-C), 139.2 (s, Ar-C), 135.7 (s, Ar-C), 135.5 (s, Ar-C), 130.3 (2 × d, 2C, Ar-CH–CH
CH), 2.39 (s, 3H, Ar-CH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 141.0 (s, Ar-C), 139.2 (s, Ar-C), 135.7 (s, Ar-C), 135.5 (s, Ar-C), 130.3 (2 × d, 2C, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar and Ar-CH), 129.9 (d, Ar-CH), 127.7 (2 × d, 2C, Ar-CH–CH
CH-Ar and Ar-CH), 129.9 (d, Ar-CH), 127.7 (2 × d, 2C, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar and Ar-CH), 127.4 (d, Ar-CH), 126.0 (d, Ar-CH), 125.9 (d, Ar-CH), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 85.5 (d, Ph-CHCH
CH-Ar and Ar-CH), 127.4 (d, Ar-CH), 126.0 (d, Ar-CH), 125.9 (d, Ar-CH), 122.0 (d, Ar-CH), 121.1 (d, Ar-CH), 85.5 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.8 (t, Ph-CH2OCHCH
CH), 72.8 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 19.9 (q, Ar-CH3) ppm. HR-MS (ESI+): m/z calculated for [C17H16NaO]+ = [M + Na]+: 259.1093; found 259.1099.
CH), 19.9 (q, Ar-CH3) ppm. HR-MS (ESI+): m/z calculated for [C17H16NaO]+ = [M + Na]+: 259.1093; found 259.1099.
1-[(E)-2-(2-Methoxyphenyl)vinyl]-1,3-dihydro-2-benzofuran (6aj). GP-3 was carried out and the product 6aj (53 mg, 86%) was furnished as a colorless viscous liquid. [TLC control (petroleum ether/ethyl acetate 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, Rf(5aj) = 0.10, Rf(6aj) = 0.70 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2904, 2838, 1489, 1461, 1244, 1028, 749, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.45 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.36–7.15 (m, 5H, Ar-H), 7.09 (d, 1H, J = 15.6 Hz, ArCH
5, Rf(5aj) = 0.10, Rf(6aj) = 0.70 UV detection)]. IR (MIR-ATR, 4000–600 cm−1): νmax = 2904, 2838, 1489, 1461, 1244, 1028, 749, 697 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.45 (dd, 1H, J = 7.8 and 1.5 Hz, Ar-H), 7.36–7.15 (m, 5H, Ar-H), 7.09 (d, 1H, J = 15.6 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.91 (d, 1H, J = 7.3 Hz, Ar-H), 6.87 (d, 1H, J = 7.3 Hz, Ar-H), 6.30 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH
CH), 6.91 (d, 1H, J = 7.3 Hz, Ar-H), 6.87 (d, 1H, J = 7.3 Hz, Ar-H), 6.30 (dd, 1H, J = 15.6 and 7.8 Hz, ArCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH
CH), 5.78 [d, 1H, J = 7.8 Hz, PhCH(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH
CH], 5.23 (dd, 1H, J = 12.2 and 2.4 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH
CH), 5.13 (d, 1H, J = 12.2 Hz, PhCHaHbOCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.86 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 156.9 (s, Ar-C), 141.2 (s, Ar-C), 139.2 (s, Ar-C), 129.4 (d, Ar-CH–CH
CH), 3.86 (s, 3H, Ar-OCH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ = 156.9 (s, Ar-C), 141.2 (s, Ar-C), 139.2 (s, Ar-C), 129.4 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 128.9 (d, Ar-CH), 127.6 (d, Ar-CH–CH
CH-Ar), 128.9 (d, Ar-CH), 127.6 (d, Ar-CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-Ar), 127.4 (d, Ar-CH), 127.1 (d, Ar-CH), 127.0 (d, Ar-CH), 125.4 (s, Ar-C), 122.1 (d, Ar-CH), 121.0 (d, Ar-CH), 120.5 (d, Ar-CH), 110.8 (d, Ar-CH), 85.9 (d, Ph-CHCH
CH-Ar), 127.4 (d, Ar-CH), 127.1 (d, Ar-CH), 127.0 (d, Ar-CH), 125.4 (s, Ar-C), 122.1 (d, Ar-CH), 121.0 (d, Ar-CH), 120.5 (d, Ar-CH), 110.8 (d, Ar-CH), 85.9 (d, Ph-CHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 72.7 (t, Ph-CH2OCHCH
CH), 72.7 (t, Ph-CH2OCHCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C17H17O2]+ = [M + H]+: 253.1223; found 253.1219.
CH), 55.4 (q, Ar-OCH3) ppm. HR-MS (ESI+): m/z calculated for [C17H17O2]+ = [M + H]+: 253.1223; found 253.1219.
Conclusions
In summary, we have developed an efficient and practical method for the direct synthesis of 1,3-dihydroisobenzofurans, an important structural motif present in biologically active natural or synthetic compounds. [Pd]-catalyzed controlled intermolecular Mizoroki–Heck coupling and reduction were performed sequentially. The direct treatment of the resultant crude diol without further purification with BF3·Et2O gave 1,3-dihydroisobenzofurans. Significantly, the method enabled the synthesis of 1,3-dihydroisobenzofurans with simple to electron rich aromatic rings. Importantly, the protocol is also applicable for a wide range of ortho substituted allylic alcohols. It is worth mentioning that although the yields of the cyclic ether 1,3-dihydroisobenzofurans are moderate, it actually represents the overall yield of three individual reactions.Acknowledgements
Council of Scientific and Industrial Research [(CSIR), 02(0018)/11/EMR-II], New Delhi, is gratefully acknowledged. JK and PN thank CSIR, New Delhi, for awarding the research fellowship.Notes and references
- (a) A.-L. Ulaczyk and D. G. Hall, Curr. Opin. Chem. Biol., 2005, 9, 266–276 CrossRef PubMed; (b) P. J. Parsons, C. S. Penkett and A. J. Shell, Chem. Rev., 1996, 96, 195–206 CrossRef CAS PubMed; (c) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed; (d) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2963 CrossRef CAS PubMed; (e) L. F. Tietze, H. Ila and H. P. Bell, Chem. Rev., 2004, 104, 3453–3516 CrossRef CAS PubMed; (f) A. Domling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed; (g) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef CAS PubMed; (h) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed; (i) B. B. Toure and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef CAS PubMed; (j) J. Le Bras and J. Muzart, Chem. Rev., 2011, 111, 1170–1214 CrossRef CAS PubMed; (k) B. Liégault, J.-L. Renaud and C. Bruneau, Chem. Soc. Rev., 2008, 37, 290–299 RSC.
- (a) X. Zhang, A. Liu and W. Chen, Org. Lett., 2008, 10, 3849–3852 CrossRef CAS PubMed; (b) J. Chae, J. Yun and S. L. Buchwald, Org. Lett., 2004, 6, 4809–4812 CrossRef CAS PubMed; (c) J. Yun and S. L. Buchwald, Org. Lett., 2001, 3, 1129–1131 CrossRef CAS PubMed; (d) B. H. Lipshutz, W. Chrisman, K. Noson, P. Papa, J. A. Sclafani, R. W. Vivian and J. M. Keith, Tetrahedron, 2000, 56, 2779–2788 CrossRef CAS; (e) C.-H. Cho, I.-S. Kim and K. Park, Tetrahedron, 2004, 60, 4589–4599 CrossRef CAS PubMed; (f) S. Paul, S. Samanta and J. K. Ray, Tetrahedron Lett., 2010, 51, 5604–5608 CrossRef CAS PubMed; (g) M. Ghosh, A. Ahmed, S. Dhara and J. K. Ray, Tetrahedron Lett., 2013, 54, 4837–4840 CrossRef CAS PubMed; (h) Y. Tian, J. Qi, C. Sun, D. Yin, X. Wang and Q. Xiao, Org. Biomol. Chem., 2013, 11, 7262–7266 RSC; (i) S. U. Son, K. H. Park and Y. K. Chung, J. Am. Chem. Soc., 2002, 124, 6838–6839 CrossRef CAS PubMed; (j) E. M. Beccalli, G. Broggini, M. Martinelli, N. Masciocchi and S. Sottocornola, Org. Lett., 2006, 8, 4521–4524 CrossRef CAS PubMed; (k) H. A. Oskooie, M. M. Heravi and F. K. Behbahani, Molecules, 2007, 12, 1438–1446 CrossRef CAS; (l) R. Sanz, V. Guilarte and M. P. Castroviejo, Synlett, 2008, 19, 3006–3010 CrossRef PubMed.
- (a) A. Bruggink, R. Schoevaart and T. Kieboom, Org. Process Res. Dev., 2003, 7, 622–640 CrossRef CAS; (b) S. P. Maddaford, N. G. Andersen, W. A. Cristofoli and B. A. Keay, J. Am. Chem. Soc., 1996, 118, 10766–10773 CrossRef CAS; (c) L. E. Overman and M. D. Rosen, Angew. Chemie., 2000, 112, 4768–4771 (Angew. Chem., Int. Ed., 2000, 39, 4596–4599) CrossRef; (d) R. Grigg, P. Fretwell, C. Meerholtz and V. Sridharan, Tetrahedron, 1994, 50, 359–370 CrossRef CAS; (e) M. R. Fielding, R. Grigg, V. Sridharan, M. Thornton-pett and C. J. Urch, Tetrahedron, 2001, 57, 7737–7748 CrossRef CAS; (f) L. Shi, C. K. Narula, K. T. Mak, L. Kao, Y. Xu and R. F. Heck, J. Org. Chem., 1983, 48, 3894–3900 CrossRef CAS; (g) G. Cuny, M. Bois-Choussy and J. Zhu, J. Am. Chem. Soc., 2004, 126, 14475–14484 CrossRef CAS PubMed; (h) H. Zhang and R. C. Larock, J. Org. Chem., 2003, 68, 5132–5138 CrossRef CAS PubMed; (i) A. Arcadi, S. Cacchi, G. Fabrizi and F. Marinelli, Synlett, 2000, 394–396 CAS; (j) G. Dyker, J. Org. Chem., 1993, 58, 6426–6428 CrossRef CAS; (k) X. Xu, Y. Qian, L. Yang and W. Hu, Chem. Commun., 2011, 47, 797–799 RSC; (l) X. Xu, W.-H. Hu, P. Y. Zavalij and M. P. Doyle, Angew. Chem., 2011, 123, 11348–11351 (Angew. Chem., Int. Ed., 2011, 50, 11152–11155) CrossRef; (m) P. Wipf, C. R. J. Stephenson and K. Okumura, J. Am. Chem. Soc., 2003, 124, 14694–14695 CrossRef PubMed; (n) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and B. Lakshmi, Acc. Chem. Res., 2003, 36, 899–907 CrossRef CAS PubMed; (o) J. Zhu, Eur. J. Org. Chem., 2003, 7, 1133–1144 CrossRef; (p) W. Hu, X. Xu, J. Zhou, W.-J. Liu, H. Huang, J. Hu, L. Yang and L.-Z. Gong, J. Am. Chem. Soc., 2008, 130, 7782–7783 CrossRef CAS PubMed; (q) P. Truong, X. Xu and M. P. Doyle, Tetrahedron Lett., 2011, 52, 2093–2096 CrossRef CAS PubMed.
- (a) A. Ahmed, S. Dhara and J. K. Ray, Tetrahedron Lett., 2013, 54, 1673–1676 CrossRef CAS PubMed; (b) J. M. A. Miguez, L. A. Adrio, A. Sousa-pedrares, J. M. Vila and K. K. Hii, J. Org. Chem., 2007, 72, 7771–7774 CrossRef CAS PubMed; (c) S. T. Handy, T. Wilson and A. Muth, J. Org. Chem., 2007, 72, 8496–8500 CrossRef CAS PubMed; (d) S. A. Springfield, K. Marcantonio, S. Ceglia, J. Albaneze-walker, P. G. Dormer, T. D. Nelson and J. A. Murry, J. Org. Chem., 2003, 68, 4598–4599 CrossRef CAS PubMed.
- (a) M. Shiri, Chem. Rev., 2012, 112, 3508–3549 CrossRef CAS PubMed; (b) H. Lebel, C. Ladjel and L. Brthous, J. Am. Chem. Soc., 2007, 129, 13321–13326 CrossRef CAS PubMed; (c) M. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art and M. Nomura, J. Org. Chem., 1998, 63, 5211–5215 CrossRef CAS.
- E.-i. Negishi, C. Copéret, S. Ma, S.-Y. Liou and F. Liu, Chem. Rev., 1996, 96, 365–393 CrossRef CAS PubMed.
- For some recent domino one-pot Pd-catalyzed transformations, see: (a) C. Chowdhury, S. Mukherjee, B. Chakraborty and B. Achari, Org. Biomol. Chem., 2011, 9, 5856–5862 RSC; (b) J. Lubkoll, A. Millemaggi, A. Perry and R. J. K. Taylor, Tetrahedron, 2010, 66, 6606–6612 CrossRef CAS PubMed; (c) M. L. N. Rao and P. Dasgupta, Tetrahedron Lett., 2012, 53, 162–165 CrossRef CAS PubMed; (d) B. Laleu and M. Lautens, J. Org. Chem., 2008, 73, 9164–9167 CrossRef CAS PubMed; (e) N. Selander and K. J. Szabó, J. Org. Chem., 2009, 74, 5695–5698 CrossRef CAS PubMed; (f) Y. Cheng, Z. Duan, L. Yu, Z. Li, Y. Zhu and Y. Wu, Org. Lett., 2008, 10, 901–904 CrossRef CAS PubMed; (g) G. Satyanarayana and M. E. Maier, Org. Lett., 2008, 10, 2361–2364 CrossRef CAS PubMed; (h) I. Kim and K. Kim, Org. Lett., 2010, 12, 2500–2503 CrossRef CAS PubMed; (i) G. Satyanarayana and M. E. Maier, Eur. J. Org. Chem., 2008, 5543–5552 CrossRef CAS; (j) O. Leogane and H. Lebel, Angew. Chem., 2008, 120, 356–358 (Angew. Chem., Int. Ed., 2008, 47, 350–352) CrossRef; (k) J. Barluenga, A. Mendoza, F. Rodríguez and J. F. Fañanás, Angew. Chem., 2009, 121, 1672–1675 (Angew. Chem., Int. Ed., 2009, 48, 1644–1647) CrossRef; (l) Y. Liang, T. Meng, H.-J. Zhang and Z. Xi, Synlett, 2011, 911–914 CAS; (m) T. Toyoshima, Y. Mikano, T. Miura and M. Murakami, Org. Lett., 2010, 12, 4584–4587 CrossRef CAS PubMed; (n) A. Schweinitz, A. Chtchemelinine and A. Orellana, Org. Lett., 2011, 13, 232–235 CrossRef CAS PubMed; (o) F. Jafarpour and N. Jalalimanesh, Tetrahedron, 2012, 68, 10286–10292 CrossRef CAS PubMed; (p) C. S. Cho, D. K. Lim, J. Q. Zhang, T.-J. Kim and S. C. Shim, Tetrahedron Lett., 2004, 45, 5653–5656 CrossRef CAS PubMed; (q) A. S. K. Hashmi, M. Ghanbari, M. Rudolph and F. Rominger, Chem.–Eur. J., 2012, 18, 8113–8119 CrossRef CAS PubMed; (r) A. S. K. Hashmi, C. Lothschütz, R. Döpp, M. Ackermann, J. D. B. Becker, M. Rudolph, C. Scholz and F. Rominger, Adv. Synth. Catal., 2012, 354, 133–137 CrossRef CAS; (s) A. S. K. Hashmi, C. Lothschütz, R. Döpp, M. Rudolph, T. D. Ramamurthi and F. Rominger, Angew. Chem., 2009, 121, 8392–8395 (Angew. Chem., Int. Ed., 2009, 48, 8243–8246) CrossRef.
- For some recent sequential one-pot Pd-catalyzed transformations, see: (a) R.-J. Song, Y. Liu, R.-J. Li and J.-H. Li, Tetrahedron Lett., 2009, 50, 3912–3916 CrossRef CAS PubMed; (b) L. Nassar-Hardy, S. Fabre, A. M. Amer, E. Fouquet and F.-X. Felpin, Tetrahedron Lett., 2012, 53, 338–341 CrossRef CAS PubMed; (c) Y. Zhou, Y. Zhao, X. Dai, J. Liu, L. Li and H. Zhang, Org. Biomol. Chem., 2011, 9, 4091–4097 RSC; (d) J.-Y. Lee and P. Ho Lee, J. Org. Chem., 2008, 73, 7413–7416 CrossRef CAS PubMed; (e) T. Matsuda, M. Shigeno and M. Murakami, Org. Lett., 2008, 10, 5219–5221 CrossRef CAS PubMed; (f) Y. Zhao, Y. Zhou, L. Liang, X. Yang, F. Du, L. Li and H. Zhang, Org. Lett., 2009, 11, 555–558 CrossRef CAS PubMed; (g) J. Peng, D. Jiang, W. Lin and Y. Chen, Org. Biomol. Chem., 2007, 5, 1391–1396 RSC.
- For reviews on domino Heck reactions, see: (a) E. Negishi, C. Copret, S. Ma, S.-Y. Liou and F. Liu, Chem. Rev., 1996, 96, 365–394 CrossRef CAS PubMed; (b) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed; (c) A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945–2964 CrossRef CAS PubMed; (d) J. Dupont, C. S. Consorti and J. Spencer, Chem. Rev., 2005, 105, 2527–2572 CrossRef CAS PubMed; (e) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644–4680 CrossRef CAS PubMed.
- For recent domino Heck cyclizations, see: (a) R. T. Ruck, M. A. Huffman, M. M. Kim, M. Shevlin, W. V. Kandur and I. W. Davies, Angew. Chem., 2008, 120, 4789–4792 (Angew. Chem., Int. Ed., 2008, 47, 4711–4714) CrossRef; (b) G. Satyanarayana, C. Maichle-Mössmerzb and M. E. Maier, Chem. Commun., 2009, 1571–1573 RSC; (c) Y. Hu, C. Yu, D. Ren, Q. Hu, L. Zhang and D. Cheng, Angew. Chem., 2009, 121, 5556–5559 (Angew. Chem., Int. Ed., 2009, 48, 5448–5451) CrossRef; (d) L. F. Tietze, A. Düfert, F. Lotz, L. Sölter, K. Oum, T. Lenzer, T. Beck and R. Herbst-Irmer, J. Am. Chem. Soc., 2009, 131, 17879–17884 CrossRef CAS PubMed.
- For recent domino Heck couplings, see: (a) O. Rene, D. Lapointe and K. Fagnou, Org. Lett., 2009, 11, 4560–4563 CrossRef CAS PubMed; (b) Z. Lu, C. Hu, J. Guo, J. Li, Y. Cui and Y. Jia, Org. Lett., 2010, 12, 480–483 CrossRef CAS PubMed; (c) M. Hussain, S.-M. T. Toguem, R. Ahmad, D. T. Tung, I. Knepper, A. Villinger and P. Langer, Tetrahedron, 2011, 67, 5304–5318 CrossRef CAS PubMed; (d) I. Ullah, M. Nawaz, A. Villinger and P. Langer, Tetrahedron Lett., 2011, 52, 1888–1890 CrossRef CAS PubMed.
- F.-X. Felpin, O. Ibarguren, L. Nassar-hardy and E. Fouquet, J. Org. Chem., 2009, 74, 1349–1352 CrossRef CAS PubMed.
- (a) A. G. K. Reddy, J. Krishna and G. Satyanarayana, Synlett, 2011, 1756–1760 CAS; (b) J. Krishna, A. G. K. Reddy, B. V Ramulu, L. Mahendar and G. Satyanarayana, Synlett, 2012, 23, 375–380 CrossRef CAS PubMed; (c) A. G. K. Reddy and G. Satyanarayana, Tetrahedron, 2012, 68, 8003–8010 CrossRef CAS PubMed; (d) A. G. K. Reddy, J. Krishna and G. Satyanarayana, Tetrahedron Lett., 2012, 53, 5635–5640 CrossRef PubMed; (e) J. Krishna, A. G. K. Reddy and G. Satyanarayana, Synlett, 2013, 24, 967–972 CrossRef CAS PubMed; (f) A. G. K. Reddy, J. Krishna and G. Satyanarayana, Tetrahedron, 2013, 69, 10098–10107 CrossRef CAS PubMed; (g) B. Suchand, J. Krishna, B. V. Ramulu, D. Dibyendu, A. G. K. Reddy, L. Mahendar and G. Satyanarayana, Tetrahedron Lett., 2012, 53, 3861–3864 CrossRef CAS PubMed; (h) B. V. Ramulu, L. Mahendar, J. Krishna, A. G. K. Reddy, B. Suchand and G. Satyanarayana, Tetrahedron, 2013, 69, 8305–8315 CrossRef CAS PubMed; (i) J. Krishna, A. G. K. Reddy and G. Satyanarayana, Tetrahedron Lett., 2014, 55, 861–864 CrossRef CAS PubMed; (j) J. Krishna, A. G. K. Reddy and G. Satyanarayana, Synth. Commun., 2014, 44, 2103–2111 CrossRef CAS; (k) L. Mahendar, J. Krishna, A. G. K. Reddy, B. V Ramulu and G. Satyanarayana, Org. Lett., 2012, 14, 628–631 CrossRef CAS PubMed; (l) L. Mahendar and G. Satyanarayana, J. Org. Chem., 2014, 79, 2059–2074 CrossRef CAS PubMed; (m) L. Mahendar, A. G. K. Reddy, J. Krishna and G. Satyanarayana, J. Org. Chem., 2014, 79, 8566–8576 CrossRef CAS PubMed.
- (a) T. Jeffery, J. Chem. Soc., Chem. Commun., 1984, 1287–1289 RSC; (b) A. Briot, C. Baehr, R. Brouillard, A. Wagner and C. Mioskowski, J. Org. Chem., 2004, 69, 1374–1377 CrossRef CAS PubMed.
- (a) U. Holler, J. B. Gloer and D. T. Wicklow, J. Nat. Prod., 2002, 65, 876–882 CrossRef PubMed; (b) J. K. Harper, A. M. Arif, E. J. Ford, G. a. Strobel, J. a. Porco, D. P. Tomer, K. L. Oneill, E. M. Heider and D. M. Grant, Tetrahedron, 2003, 59, 2471–2476 CrossRef CAS; (c) Y.-J. Kwon, C.-J. Zheng and W.-G. Kim, Biosci., Biotechnol., Biochem., 2010, 74, 390–393 CrossRef CAS PubMed; (d) Y. Nishihara, S. Takase, E. Tsujii, H. Hatanaka and S. Hashimoto, J. Antibiot., 2001, 54, 297–303 CrossRef CAS; (e) Y. Nishihara, E. Tsujii, Y. Yamagishi, K. Sakamoto, Y. Tsurumi, S. Furukawa, M. Hino, M. Yamashita and S. Hasimoto, J. Antibiot., 2001, 54, 136–143 CrossRef CAS; (f) Z. P. Xie, H. Y. Zhang, F. C. Li, B. Liu, S. X. Yang, H. P. Wang, Y. Pu, Y. Chen and S. Qin, Chin. Chem. Lett., 2012, 23, 941–944 CrossRef CAS PubMed; (g) D. Shi, X. Fan, L. Han, F. Xu and Z. Yuan, Faming Zhuanli Shenqing Gongkai Shuomingshu, CN Pat., 101283998 A, 15 Oct 2008; (h) K. Dorell, M. A. Cohen, S. S. Huprikar, J. M. Gorman and M. Jones, Psychosomatics, 2005, 46, 91–93 CrossRef PubMed; (i) N. G. Parker and C. S. Brown, Ann. Pharmacother., 2000, 34, 761–771 CAS; (j) K. Brøsen and C. A. Naranja, Eur. Neuropsychopharmacol., 2001, 11, 275–283 CrossRef; (k) D. Baldwin and F. N. Johnson, Rev. Contemp. Pharmacother., 1995, 6, 315–325 CAS; (l) M. B. Keller and J. Clin, Psychiatry, 2000, 61, 896–908 CAS.
- R. Karmakar, P. Pahari and D. Mal, Chem. Rev., 2014, 114, 6213–6284 CrossRef CAS PubMed.
- S. Chandrasekhar, N. R. Reddy and Y. S. Rao, Tetrahedron, 2006, 62, 12098–12107 CrossRef CAS PubMed.
- H. Muratake, M. Natsume and H. Nakai, Tetrahedron, 2004, 60, 11783–11803 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00955c |
| This journal is © The Royal Society of Chemistry 2015 |






