The effect of the aliphatic carboxylate linkers on the electronic structures, chemical bonding and optical properties of the uranium-based metal–organic frameworks†
Abstract
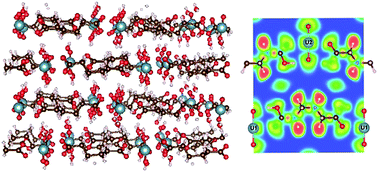
Metal–organic frameworks (MOFs) are drawing increased interest for their high stability and porosity leading to their potential applications in separation, catalysis, and photoelectric processes. Recent studies have identified the role of aminated linkers in band-gap reduction of different MOFs. Uranyl-containing MOFs are of particular interest due to their photo-luminescent properties and their photocatalytic activity. We recently studied the electronic structures of a series of uranyl containing aliphatic dicarboxylate structures that contain aliphatic dicarboxylate linkers of different lengths. Members of this series are UO2–C4H4O4–H2O (MOF1), UO2–C5H6O4 (MOF2), UO2–C6H8O4–2H2O (MOF3), UO2–C7H10O4 (MOF4), UO2–C8H12O4 (MOF5), UO2–C9H14O4 (MOF6), and UO2–C10H6O4 (MOF7). This series of actinide coordination polymers were synthesized by various groups. Our computational study provides a detail analysis of chemical bonding, charge distribution, geometric and electronic structural properties, and optical properties of these MOFs for the first time. The variation in the length of linkers does not significantly influence the electronic properties of these MOFs. All MOFs of this series show semiconducting character, common in other transition metal-based MOFs. The band gap for the whole series is essentially constant at ca. 2.5 eV, independent of the length of linkers. For the first time, we provide an extensive analysis of the bonding environment and characteristics in these MOFs based on the charge density distribution, Bader and Mulliken population analysis as well as electron localization functions (ELF). Our analyses show that the uranyl metallic subunit in all MOFs has ionic bonding characteristics. The organic carboxylate linkers, on the other hand, show predominantly covalent bonding characteristics. Simulated optical properties, such as refractive index n(ω), absorption coefficient α(ω), optical conductivity σ(ω), reflectivity R(ω), and electron energy-loss spectrum L(ω) are obtained from the calculated frequency dependent dielectric constants. These properties indicate some promising application of these MOFs as photo-catalyst. In particular, substantial absorption in the energy range of the visible part of electromagnetic spectrum shown by these MOFs can be explored further for application in solar energy sector. Our results also indicate to a possible way to tune the band gap through, e.g., doping, and hence pave the path to a potential application of these MOFs as photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...