Catecholamine toxicity triggers myocardial membrane destabilization in rats: thymol and its counter action
Mohamed Fizur Nagoor Meeran,
Govindan Sangaran Jagadeesh and
Palanisamy Selvaraj*
Department of Biochemistry and Biotechnology, Annamalai University, Annamalai Nagar-608 002, Tamil Nadu, India. E-mail: drselvarajau@gmail.com; Tel: +91 9865434202
First published on 15th April 2015
Abstract
We evaluated the protective effects of thymol on myocardial membrane destabilization by isoproterenol (ISO), a synthetic catecholamine which triggers cardiotoxicity in rats. Male albino Wistar rats were pre and co-treated with thymol (7.5 mg kg−1) daily for 7 days. ISO (100 mg kg−1) was injected subcutaneously into rats at an interval of 24 h for two days (6th and 7th days) to induce cardiotoxicity. Significantly increased levels/activity of cardiac troponin-T and lactate dehydrogenase (LDH) with increased concentrations of heart thiobarbituric acid reactive substances (TBARS), and decreased concentrations of superoxide dismutase and catalase, were observed in ISO induced cardiotoxic rats. The activity of sodium/potassium-dependent adenosine triphosphatase (Na+/K+-ATPase) was significantly decreased and the activities of calcium and magnesium-dependent adenosine triphosphatases (Ca2+-ATPase and Mg2+-ATPase) were significantly increased in the hearts of ISO induced cardiotoxic rats. Furthermore, ISO induced cardiotoxic rats also showed decreased concentrations of potassium (K+) and increased concentrations of sodium (Na+) and calcium (Ca2+) in the heart. Pre and co-treatment with thymol showed near normalized effects on all the biochemical parameters studied. Also, thymol greatly reduced myocardial infarct size. Thus, the present study revealed that thymol prevented the myocardial membrane destabilization in ISO induced cardiotoxic rats due to its strong membrane stabilizing properties.
1. Introduction
Cardiovascular diseases (CVDs) have a high prevalence in developing and developed countries An approach used for inducing cardiotoxicity in animal models is to administer a large dose of ISO. It is a synthetic catecholamine and a β-adrenergic agonist which causes severe stress in the myocardium, resulting in infarct-like necrosis of the heart muscle.1The free radicals produced by ISO could initiate the peroxidation of membrane bound polyunsaturated fatty acids leading to both functional and structural cardiotoxicity.2 The myocardial membrane destabilization in experimental animals by ISO may also be due to its action on the sarcolemmal membrane, stimulation of adenylate cyclase, activation of Na+ and Ca2+ channels, exaggregated Ca2+ inflow and energy consumption leading to cellular death.3
ATPases of cardiac cells play a significant role in the contraction and relaxation cycles of cardiac muscle by maintaining normal ion levels (Na+, K+ and Ca2+) within the myocytes.1 Alterations in the properties of these ion pumps may affect cardiac function. They act as key messengers in signal transduction pathways in the heart and hence the regulation of cardiac contractility and hypertrophy.1 Cellular injury is associated with alterations in mineral homeostasis. Thus, these enzymes and minerals play a vital role in the pathology of cardiotoxicity.4 These ATPases offer potentially exciting opportunities as new therapeutic targets for cardiotoxicity and heart failure.5
Thyme and oregano have been commonly used in foods, mainly for enhancing their flavor, aroma and preservation and also in folk medicine since the time of the ancient Greeks, Egyptians and Romans. The leafy parts of thyme belonging to the Lamiaceae family are often added to meat, fish and fish products and also used as herbal medicinal products.6 Thyme essential oil and its ingredients have been shown to exhibit a wide range of biological properties.7
Thymol, an active compound and a dietary monoterpene phenol, which is found in the oils of thyme and plants such as Thymus vulgaris, Thymbra spicata, Thymus ciliates, Origanum vulgarae, and Trachyspermum ammi species, as well as Monarda fistulosa and Nigella sativa seeds. Thymol also exhibits antibacterial,8 antifungal,9 anti-inflammatory10 and radioprotective activities.11 Furthermore, it is rapidly absorbed after oral administration in humans and the maximum plasma concentration is reached after 1.97 h.12
A preliminary dose dependent study revealed the protective effects of thymol on altered serum creatine kinase-MB, plasma lipid peroxidation products and plasma non-enzymatic antioxidants in ISO induced myocardial infarcted Wistar rats.13 In continuation of our research on thymol, in this investigation, we evaluated the protective effects of thymol on myocardial membrane destabilization due to enhanced lipid peroxidation and changes in the activities/concentrations of ATPases and minerals in the hearts of rats with ISO induced cardiotoxicity.
2. Materials and methods
2.1. Drugs and chemicals
Thymol and isoproterenol hydrochloride were purchased from Sigma Chemical Company, St. Louis, MO. Thiobarbituric acid, xylenol orange, trichloro acetic acid, adenosine triphosphate, amino napthol sulfonic acid, 2,3,5 triphenyl tetrazolium chloride, hydrochloric acid, magnesium sulphate, potassium chloride and magnesium chloride were obtained from S.D. Fine Chemicals, Mumbai, India. All the other chemicals used were of analytical grade.2.2. Experimental animals
All the experiments were done with male albino Wistar rats weighing 160–180 g, aged 7–8 weeks old obtained from Central Animal House, Rajah Muthiah Institute of Health Sciences, Annamalai University, Tamil Nadu, India. The experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi, India. The experimental protocol was approved by the Animal Ethical Committee of Annamalai University (proposal no. 835; approval date: September 28, 2011). Rats were housed in polypropylene cages (47 × 34 × 20 cm) lined with husk (replaced every 24 h) in a 12 h light–dark cycle at around 22 °C. Rats were fed on standard pelleted feed (Pranav Agro Industries Ltd, Pune, Maharashtra, India) and water ad libitum.2.3. Induction of cardiotoxicity in Wistar rats
ISO (100 mg kg−1 body weight) dissolved in saline was subcutaneously injected into rats at an interval of 24 h for 2 days.14,15 ISO induced cardiotoxicity was confirmed by the elevated levels/activity of serum cardiac troponin-T and LDH in rats.2.4. Experimental design
The animals were grouped into four groups of six rats each. Group-I: normal control rats; Group-II: rats were orally treated with thymol (7.5 mg kg−1 body weight) dissolved in 0.5% dimethyl sulfoxide (DMSO) daily for a period of 7 days by an intragastric tube; Group-III: rats were subcutaneously injected with ISO (100 mg kg−1 body weight) at an interval of 24 h for 2 days (6th and 7th days) to induce cardiotoxicity; Group-IV: rats were orally pre and co-treated with thymol (7.5 mg kg−1 body weight) dissolved in 0.5% DMSO daily for a period of 7 days by an intragastric tube and were subcutaneously injected with ISO at an interval of 24 h for two days (6th and 7th days). Normal control rats and ISO control rats were given 0.5% DMSO alone orally daily for a period of 7 days by an intragastric tube. The dosage (7.5 mg kg−1 body weight) and duration of pre and co-treatment of thymol (7 days) were fixed based on our earlier study.13 Twelve hours after the second dose of ISO injection (8th day), all the rats were anesthetized and then sacrificed by cervical decapitation. For serum, blood was collected in dry tubes without anticoagulant. Heart tissues were excised immediately and rinsed in ice-chilled saline. Known weights of the tissues were homogenized in 5.0 ml of 0.1 M Tris–HCl buffer (pH-7.4) and used for the various biochemical estimations. All enzyme assays were done immediately.2.5. In vivo studies
| IS-CP (%) = [(IA1/TA1 × wt1) + ((IA2/TA2 × wt2) + … + (IAi/TAi × wti)]/(wt1 + wt2 + … wt3) |
2.6. Estimation of protein content in the heart
The protein content in the tissue homogenates was estimated by the method of Lowry et al.242.7. Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using Statistical Package for the Social Sciences (SPSS) software package version 12.00. Results were expressed as mean ± Standard deviation (S.D.) for six rats in each group. P values <0.05 were considered significant.3. Results
ISO induced cardiotoxic rats (Group-III) showed a significant (P < 0.05) increase in the level of serum cardiac troponin-T (2.41 ± 0.2 ng ml−1) compared to normal control rats (0.31 ± 0.03 ng ml−1) (Group-I). Pre and co-treatment with thymol (7.5 mg kg−1 body weight) daily for a period of 7 days near normalized the levels of serum cardiac troponin-T (0.44 ± 0.03 ng ml−1) in ISO induced cardiotoxic rats (Group-IV) compared to ISO-alone induced cardiotoxic rats (Group-III). Rats treated with thymol (0.28 ± 0.03 ng ml−1) did not show any significant change in serum cardiac troponin-T levels (Group-II).The rats induced with ISO showed a significant (P < 0.05) increase in the activity of serum LDH when compared to normal control rats. Pre and co-treatment with thymol near normalized the activity of LDH in the serum of ISO induced cardiotoxic rats compared to ISO-alone induced cardiotoxic rats (Fig. 1).
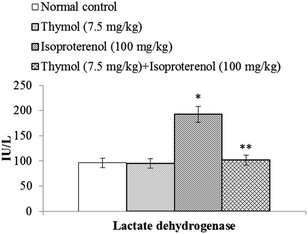 | ||
| Fig. 1 Activity of LDH in the serum. Each column is mean ± S.D. for six rats in each group; columns not sharing a common symbol (* and **) differ significantly with each other (P < 0.05; DMRT). | ||
ISO induced cardiotoxic rats showed a significant (P < 0.05) increase in the concentrations of TBARS in the heart tissue homogenate when compared to normal control rats. Pre and co-treatment with thymol near normalized the concentrations of TBARS in the hearts of ISO induced cardiotoxic rats when compared to ISO-alone induced cardiotoxic rats (Fig. 2).
ISO induced cardiotoxic rats showed a significant (P < 0.05) decrease in the activities of superoxide dismutase and catalase in the heart tissue homogenate when compared to normal control rats. Pre and co-treatment with thymol near normalized the activities of superoxide dismutase and catalase in the hearts of ISO induced cardiotoxic rats when compared to ISO-alone induced cardiotoxic rats (Fig. 3).
The activity of Na+/K+-ATPase was significantly (P < 0.05) decreased and the activities of Ca2+-ATPase and Mg2+-ATPase were significantly (P < 0.05) increased in the hearts of ISO induced cardiotoxic rats compared to normal control rats. Pre and co-treatment with thymol revealed near normalized activities of ATPases in the hearts of ISO induced cardiotoxic rats when compared to ISO-alone induced cardiotoxic rats (Fig. 4 and 5).
Fig. 6 illustrates the effect of thymol on the concentrations of minerals such as Na+, K+ and Ca2+ ions in the hearts of normal and ISO induced cardiotoxic rats. ISO induced cardiotoxic rats showed a significant (P < 0.05) increase in the concentrations of Na+ and Ca2+ ions with a significant (P < 0.05) decrease in the levels of K+ ions in the heart when compared to normal control rats. Pre and co-treatment of ISO induced cardiotoxic rats with thymol near normalized the concentrations of Na+, K+ and Ca2+ in the heart as compared to ISO-alone induced cardiotoxic rats.
The size of the myocardial infarct determined by the TTC test shows the ISO induced cardiotoxicity in rats. Fig. 7(A–D) show images of heart slices after staining with TTC. Fig. 7A is the TTC stained heart slice of a normal rat, showing completely viable tissue without infarction. Fig. 7B is the heart slice of a thymol (7.5 mg kg−1 body weight) alone treated rat stained with TTC, showing completely viable myocardial tissue without any infarction. Fig. 7C is the section of a heart of an ISO (100 mg kg−1 body weight) induced cardiotoxic rat. Infarcted tissues are clearly visible and colourless. Infarcted tissues did not stain with TTC because there is leakage of LDH enzyme from that area. Fig. 7D is the heart slice of a rat pre and co-treated with thymol (7.5 mg kg−1 body weight) induced with ISO and stained with TTC, showing greatly reduced myocardial infarct size. It is clear from Fig. 7D that pre and co-treatment with thymol of ISO induced cardiotoxic rats might have prevented membrane damage caused by ISO, thereby reducing myocardial infarct size and maintaining the stability of the myocardial membrane.
Fig. 8 shows the infarct size quantified by a cumulative planimetry method by using the suitable formula given in the methodology. ISO induced cardiotoxic rats (Group-III) showed an increase in % infarct regions (52%) in cumulative planimetry analysis, while pre and co-treatment with thymol significantly (P < 0.05) reduced % infarct region (16%) in ISO induced cardiotoxic rats (Group-IV). Normal control rats (Group-I) and rats treated with thymol (Group-II) did not show any infarct regions (0%).
Table 1 represents the effect of thymol on the body weight and heart weight of normal and ISO induced cardiotoxic rats. ISO induced rats showed no significant changes in the body weight but revealed considerably increased heart weight. Thymol pre and co-treated cardiotoxic rats showed a considerable decrease in heart weight compared with ISO induced cardiotoxic rats.
| Groups | Normal control | Thymol (7.5 mg kg−1) | ISO (100 mg kg−1) | Thymol (7.5 mg kg−1) + ISO (100 mg kg−1) |
|---|---|---|---|---|
| a P < 0.05 as compared to normal control (Group-I).b P < 0.05 as compared to ISO control (Group-III) (DMRT). | ||||
| Heart weight (mg) | 511.1 ± 50.2 | 510.4 ± 49.8 | 867.5 ± 81.2a | 617.3 ± 60.0b |
| Body weight (g) | 171.4 ± 16.9 | 170.4 ± 16.9 | 171.1 ± 17.0 | 172.3 ± 17.1 |
For the all biochemical parameters studied, thymol (7.5 mg kg−1 body weight) alone treated rats (Group-II) did not show any significant effect compared to normal control rats (Group-I).
4. Discussion
To the best of our knowledge, this is the first study carried out on the protective effects of thymol on membrane destabilization in ISO induced cardiotoxic rats. In this study the mechanisms of thymol in ISO induced cardiotoxicity were also further investigated.Troponin-T is one of the myocardial tissue specific proteins of the troponin regulatory complex, and is a highly specific and sensitive marker in the determination of myocardial cell injury. It is a powerful biomarker in laboratory animals for sensitive and specific detection of cardiac injury arising from various causes.25 It is a contractile protein that is normally not found in the serum. It is released only when myocardial necrosis occurs. Elevated cardiac troponin-T levels in the serum predict the risk of both cardiac death and subsequent infarction. Pre and co-treatment with thymol (7.5 mg kg−1 body weight) near normalized the levels of cardiac troponin-T in the serum of ISO induced cardiotoxic rats. This effect could be due to the cardioprotective properties of thymol on myocardium, maintaining membrane stability and integrity thereby restricting the leakage of cardiac troponin-T into the circulation.
Myocardium contains plentiful concentrations of diagnostic markers of MI and once metabolically damaged, it releases its contents into the extracellular fluid.26 LDH is one of the diagnostic markers of myocyte injury or death and is found to be increased in the serum of ISO induced cardiotoxic rats. Due to poor oxygen or glucose supply the myocardial cells are damaged, making the cardiac membrane permeable resulting in the leakage of LDH into the serum. This might be due to the damage caused to the sarcolemma by the β-adrenergic agonist, which has rendered it leaky. Pre and co-treatment with thymol near normalized the activities of LDH in the serum of ISO induced cardiotoxic rats. Thymol, due to its potent free radical scavenging and membrane stabilizing activity, defends the myocardium from destruction by inhibiting LDH enzyme leakage into the circulation in ISO induced cardiotoxic rats.
Lipid peroxidation, a type of oxidative deterioration of polyunsaturated fatty acids, has been linked with altered membrane structure and enzyme inactivation. Increased lipid peroxidation products in ISO induced cardiotoxic rats appear to be an initial stage of damage to the tissue, making it more susceptible to oxidative damage.27 Increased free radical production may be responsible for the observed myocardial membrane destabilization as evidenced by the elevated lipid peroxidation in terms of TBARS in the hearts of ISO induced cardiotoxic rats. Thymol pre and co-treatment near normalized the concentrations of TBARS in the hearts of ISO induced cardiotoxic rats. Thus, thymol inhibits lipid peroxidation by scavenging excess free radicals produced by ISO and protects the integrity of the myocardial membrane by virtue of its potent free radical scavenging, anti lipid peroxidative and membrane stabilizing properties.
Superoxide dismutase protects the myocardial cells from oxidative damage by converting superoxide radicals into hydrogen peroxide, which is further metabolized by catalase to molecular oxygen and water.28 The decreased activities of these antioxidant enzymes might be due to the myocardial cell damage. Superoxide radicals generated at the site of damage modulate superoxide dismutase and catalase resulting in the decreased activities of these enzymes and accumulation of superoxide anions, which also damage the myocardium.28 Pre and co-treatment with thymol near normalized the activities of superoxide dismutase and catalase by its potent free radical scavenging and antioxidant effects.
Membrane proteins are in general regarded as extremely potent drug targets due to their role as transporters and mediators in the interaction of cells with the surrounding environment, and between cells and cellular compartments. This role and their highly exposed position in the cell, renders them of utmost importance in cellular physiology.29
A significantly decreased activity of Na+/K+-ATPase and significantly increased activities of Ca2+-ATPase and Mg2+-ATPase with altered mineral concentrations in the heart were observed in ISO induced cardiotoxic rats. Na+/K+-ATPase, a member of the P-type ATPase family is involved in the regulation of cell volume, development of membrane potential, and transport of nutrients in animal tissues.30 The Na+/Ca2+-exchanger removes cytosolic Ca2+ rapidly in cardiomyocytes.31 This can result in disturbances in Ca2+ signaling, ventricular performance and contractility. Decreased activity of Na+/K+-ATPase could be due to enhanced lipid peroxidation by free radicals on ISO induction, since Na+/K+-ATPase is a thiol group containing enzyme and is lipid dependent.32 Elevation in the level of myocardial free fatty acids has been previously reported to result in the inhibition of several enzyme systems non-competitively such as Na+/K+-ATPase.33 We have already reported a significant increase in the levels of free fatty acids due to increased lipolysis in ISO induced cardiotoxic rats.15 Thus, the elevated levels of free fatty acids might have resulted in non-competitive inhibition of Na+/K+-ATPase thereby leading to increased accumulation of Na+ ions in ISO induced cardiotoxic rats. This can lead to a decrease in Na+ efflux, thereby altering membrane permeability.34 Ca2+-ATPase regulates the Ca2+ pump activity.35 During β-adrenergic stimulation cyclic adenosine monophosphate phosphorylates several sites on the C-terminal chains of the Ca2+ channel and increases the probability of the Ca2+ channel opening.36 This is the reason for the enhanced activity of Ca2+-ATPase and increased concentration of Ca2+ observed in the myocardial tissue of ISO induced cardiotoxic rats. Intracellular Ca2+ overload can set off a cascade of events that can lead to the formation of reactive oxygen species, which suggests that reactive oxygen species formation and calcium surge may be involved in the contractile dysfunction of the ischemic myocardium.37
Mg2+-ATPase is believed to be responsible for the aminophospholipid translocase activity of the plasma membrane,38 which could affect the activity of membrane Ca2+-ATPase.39 Also, in a previous report, a decrease in the adenosine triphosphate (ATP) content was observed in ISO induced cardiotoxic rats.40 As ATP breaks down, opening of the K+ channel is promoted41 leading to the decreased concentration of K+ in the heart tissue. Pre and co-treatment with thymol near normalized the activities/concentrations of ATPases and minerals in ISO induced cardiotoxic rats. Restoration of Na+/K+-ATPase activity due to thymol pre and co-treatment in ISO induced cardiotoxic rats could regulate the intracellular Ca2+ concentrations, thereby protecting the myocardium from excess damage by maintaining the membrane integrity. This depressed concentrations of Ca2+ due to the elevated concentrations of Na+ ions during thymol pre and co-treatment in ISO induced cardiotoxic rats due to the ability of thymol to protect the thiol groups from oxidative damage through the inhibition of peroxidation of membrane lipids. This effect is due to the membrane stabilizing property of thymol which might be due to the inhibition of lipid peroxidation in cell membranes.
TTC staining is a well-accepted method to determine myocardial infarct size, which provides a reliable index of necrosis.42 The extent of myocardial necrosis is detected by direct staining with TTC dye, which forms a red formazan precipitate in the presence of LDH, whereas the infracted myocardium lacks the activity of this enzyme and therefore fails to stain with it. The area of infarction may relate to LDH leakage and loss of membrane integrity.43 ISO induced rat hearts showed increased myocardial infarct size with less TTC absorbing capacity, thus showing a significant leakage of LDH as compared to normal control rats. Pre and co-treatment with thymol greatly reduced the myocardial infarct size with increased TTC absorbing capability, thus indicating a mild leakage of LDH as compared to normal control rats. Thus, thymol prevented membrane damage and decreased myocardial infarct size and protected the heart against ISO induced myocardial membrane destabilization in rats.
An increase in the weight of the heart observed in ISO induced myocardial infarcted rats indicates cardiac hypertrophy. The observed excessive heart weight is due to increased water content, edematous intramuscular space and extensive necrosis of cardiac muscle fibers followed by invasion of the damaged tissues by inflammatory cells.15 Pre and co-treatment with thymol decreased the heart weight and prevented cardiac hypertrophy in ISO induced myocardial infarcted rats.
5. Conclusions
In conclusion, our in vivo and in vitro findings showed that thymol (7.5 mg kg−1 body weight) protected membrane integrity and attenuates myocardial membrane destabilization by inhibiting cardiac marker enzyme leakage, maintaining lipid peroxidation, antioxidant system and reinstating the activities/concentrations of ATPases and minerals in ISO induced cardiotoxic rats, which might be due to its free radical scavenging and membrane stabilizing effects. Thymol also greatly reduced myocardial infarct size. Thymol (7.5 mg kg−1 body weight) administration to normal rats had no effects on the biochemical parameters and myocardial infarct size evaluated. Furthermore, oral intake of thyme tea and black cumin seeds may be beneficial for the prevention of cardiotoxicity. However, clinical trials are necessary to promote the use of thymol for the prevention of heart ailments in humans.References
- M. F. Nagoor Meeran and P. Stanely Mainzen Prince, J. Biochem. Mol. Toxicol., 2012, 26, 276–281 CrossRef CAS PubMed.
- P. Stanely Mainzen Prince, S. Rajakumar and K. Dhanasekar, Eur. J. Pharmacol., 2011, 688, 233–240 CrossRef PubMed.
- P. T. Devika and P. Stanely Mainzen Prince, Chem. Biol. Interact., 2008, 172, 245–252 CrossRef CAS PubMed.
- V. R. Punithavathi and P. Stanely Mainzen Prince, Life Sci., 2010, 86, 178–184 CrossRef CAS PubMed.
- D. Oceandy, P. J. Stanley, E. J. Cartwright and L. Neyses, Biochem. Soc. Trans., 2007, 35, 927–930 CrossRef CAS PubMed.
- U. Undeger, A. Basaran, G. H. Degen and N. Basaran, Food Chem. Toxicol., 2009, 47, 2037–2043 CrossRef CAS PubMed.
- E. Basch, C. Ulbricht, P. Hammerness, A. Bevins and D. Sollars, J. Herb. Pharmacother., 2004, 4, 49–67 CrossRef PubMed.
- A. Wattanasatcha, S. Rengpipat and S. Wanichwecharungruang, Int. J. Pharm., 2012, 434, 360–365 CrossRef CAS PubMed.
- P. S. Chavan and S. G. Tupe, Food Control, 2014, 46, 115–120 CrossRef CAS PubMed.
- K. R. Riella, R. R. Marinho, J. S. Santos, R. N. Pereira-Filho, J. C. Cardoso, R. L. C. Albuquerque-Junior and S. M. Thomazzi, J. Ethnopharmacol., 2012, 143, 656–663 CrossRef CAS PubMed.
- P. R. Archana, B. N. Rao and B. S. S. Rao, Mutat. Res., 2011, 726, 136–145 Search PubMed.
- C. Kohlert, G. Schindler, R. W. Marz, G. Abel, B. Brinkhaus, H. Derendorf, E. U. Grafe and M. Veit, J. Clin. Pharmacol., 2002, 42, 731–737 CrossRef CAS PubMed.
- M. F. Nagoor Meeran and P. Stanely Mainzen Prince, J. Biochem. Mol. Toxicol., 2012, 26, 368–373 CrossRef CAS PubMed.
- M. Mari Kannan and S. Darlin Quine, Eur. J. Pharmacol., 2011, 659, 45–52 CrossRef PubMed.
- M. F. Nagoor Meeran, P. Stanely Mainzen Prince and R. Hidhayath Basha, Eur. J. Pharmacol., 2012, 677, 116–122 CrossRef PubMed.
- C. G. Fraga, B. E. Leibovitz and A. L. Tappel, Free Radical Biol. Med., 1988, 4, 155–161 CrossRef CAS.
- P. Kakkar, B. Das and P. N. Viswanathan, Indian J. Biochem. Biophys., 1984, 21, 130–132 CAS.
- A. K. Sinha, Anal. Biochem., 1972, 47, 389–394 CrossRef CAS.
- S. L. Bonting, Presence of enzyme systems in mammalian tissues, Membrane and ion transport, ed. E. E. Bittar, Wiley Interscience, London, 1970, pp. 257–263 Search PubMed.
- S. Hjerten and H. Pan, Biochim. Biophys. Acta, 1983, 728, 281–288 CrossRef CAS.
- T. Ohnishi, T. Suzuki, Y. Suzuki and K. Ozawa, Biochim. Biophys. Acta, 1982, 684, 67–74 CrossRef CAS.
- J. T. Lie, P. C. Pairolero, K. E. Holley and J. L. Titus, J. Thorac. Cardiovasc. Surg., 1975, 69, 599–605 CAS.
- M. L. Reiss, S. S. Rhodes, D. F. Stowe, M. Aldakkak and A. K. S. Kamara, J. Pharmacol. Toxicol. Methods, 2009, 60, 275–280 CrossRef PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- P. J. O’Brien, G. W. Dameron, M. L. Beck, Y. J. Kang, B. K. Erickson, T. H. Di Battista, K. E. Miller, K. N. Jackson and S. Mittelstadt, Lab. Anim. Sci., 1997, 47, 486–495 Search PubMed.
- A. Upaganlawar, C. Gandhi and R. Balaraman, Plant Foods Hum. Nutr., 2009, 64, 75–80 CrossRef CAS PubMed.
- P. Stanely Mainzen Prince, H. Priscilla and P. T. Devika, Eur. J. Pharmacol., 2009, 139, 139–143 CrossRef PubMed.
- M. Rajadurai and P. Stanely Mainzen Prince, Toxicology, 2006, 228, 259–268 CrossRef CAS PubMed.
- L. Yatime, M. J. Buch-Pedersen, M. Musgaard, J. P. Morth, A. M. L. Winther, P. B. Pedersen, C. Olesen, J. P. Andersen, B. Vilsen, B. Schiett, M. G. Palmgren, J. V. Moller, P. Nissen and N. Fedosova, P-type ATPases as drug targets: tools for medicine and science, Biochim. Biophys. Acta, 2009, 1787, 207–220 CrossRef CAS PubMed.
- J. H. Kaplan, Annu. Rev. Biochem., 2002, 71, 511–535 CrossRef CAS PubMed.
- M. Egger and E. Niggli, J. Membr. Biol., 1999, 168, 107–130 CrossRef CAS.
- A. P. Ithayarasi and C. S. Devi, Indian J. Physiol. Pharmacol., 1997, 41, 369–376 CAS.
- K. Ahmed and B. S. Thomas, J. Biol. Chem., 1971, 246, 103–109 CAS.
- P. Finotti and P. Palatini, Diabetologia, 1986, 29, 623–628 CrossRef CAS.
- J. Levy, D. Rempinski and T. H. Kuo, Metabolism, 1994, 43, 604–613 CrossRef CAS.
- G. Varadi, Y. Mori, G. Mikala and A. Schwartz, Trends Pharmacol. Sci., 1995, 16, 43–49 CrossRef CAS.
- K. C. Jan and E. C. Shu, Chang Gung Med. J., 2005, 28, 369–376 Search PubMed.
- W. P. Vermeulen, J. J. Briede, G. Bunt, J. A. Op den Kamp, R. J. Kraaijenhagen and B. Roelofsen, Br. J. Haematol., 1995, 90, 56–64 CrossRef CAS PubMed.
- W. J. Strittmatter, F. Hirata and J. Axelrod, Biochem. Biophys. Res. Commun., 1979, 88, 147–153 CrossRef CAS.
- K. Senthil Kumaran and P. Stanely Mainzen Prince, J. Biochem. Mol. Toxicol., 2011, 25, 60–67 CrossRef CAS PubMed.
- J. M. Ferrero, J. Saiz, J. M. Ferrero and N. V. Thakor, Circ. Res., 1996, 79, 208–221 CrossRef CAS.
- K. Senthil Kumaran and P. Stanely Mainzen Prince, Cell Stress Chaperones, 2010, 15, 791–806 CrossRef PubMed.
- S. Panda, A. Kar, T. Banerjee and N. Sharma, Cardiovasc. Toxicol., 2012, 12, 235–242 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |







