Copper–silver oxide nanowires grown on an alloy electrode as an efficient electrocatalyst for water oxidation
Xing Huangab,
Minghao Xiea,
Yihan Chena,
Qingshuang Zonga,
Ziyu Liua and
Yong Jin*a
aDepartment of Materials Science and Engineering, Sichuan University, Chengdu 610064, China. E-mail: yongjin-scu@163.com
bCollege of Aerospace and Materials Engineering, National University of Defense Technology, Changsha 410073, China
First published on 6th March 2015
Abstract
Copper–silver oxide nanowires (CuO–Ag2O NWs) are obtained by a simple preparation method of immersing a CuAgZn alloy in a Na2O2 aqueous solution and annealing at 300 °C in air. The nanowires are used as an electrocatalyst for the oxygen evolution reaction (OER) in an alkaline aqueous solution. Through contrasting with several compositions of the alloy, we observe the best electrochemical performance from a specific metal oxide grown on CuAgZn with a mass ratio of 47%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28%
28%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25%, which displays an overpotential of 0.448 ± 0.02 V at 20 mA cm−2 and 0.475 ± 0.01 V at 30 mA cm−2, as well as a Tafel slope of 118 ± 4 mV dec−1. Copper–silver oxide nanoparticles, copper oxide nanoparticles, and silver oxide nanoparticles (CuO–Ag2O NPs, CuO NPs and Ag2O NPs) are also prepared to compare with CuO–Ag2O NWs. The results indicate the as-prepared CuO–Ag2O NWs show an improved activity for the OER. Furthermore, the prepared CuO–Ag2O NWs show good stability, where the normalized current density can remain at over 80% after the electrolysis of 10
25%, which displays an overpotential of 0.448 ± 0.02 V at 20 mA cm−2 and 0.475 ± 0.01 V at 30 mA cm−2, as well as a Tafel slope of 118 ± 4 mV dec−1. Copper–silver oxide nanoparticles, copper oxide nanoparticles, and silver oxide nanoparticles (CuO–Ag2O NPs, CuO NPs and Ag2O NPs) are also prepared to compare with CuO–Ag2O NWs. The results indicate the as-prepared CuO–Ag2O NWs show an improved activity for the OER. Furthermore, the prepared CuO–Ag2O NWs show good stability, where the normalized current density can remain at over 80% after the electrolysis of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s. This strategy for the fabrication of CuO–Ag2O NWs is a promising and facile way to develop more multi-metal oxide catalysts for energy conversion.
000 s. This strategy for the fabrication of CuO–Ag2O NWs is a promising and facile way to develop more multi-metal oxide catalysts for energy conversion.
1 Introduction
The oxygen evolution reaction (OER) is the process of generating molecular oxygen through electrochemical oxidation of water, and it is also a key determination of many energy conversion and storage systems.1–9 As a half reaction of water splitting, the OER process can be demonstrated as follows:10| 2H2O(l) → O2(g) + 4H+(aq) + 4e− | (1) |
| E° (O2/H2O) = 1.23 V versus NHE | (2) |
The rate of the oxygen evolution reaction is often limited by the activity of O–H bond breakage and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond formation.11–14 To accelerate the rate of the two procedures, catalysts with high catalytic performance for the OER should be developed and it may be a great challenge in the system of efficient energy conversion.
O bond formation.11–14 To accelerate the rate of the two procedures, catalysts with high catalytic performance for the OER should be developed and it may be a great challenge in the system of efficient energy conversion.
High current density at low over potential is the vital parameter of the efficient catalyst.15 Transition-metal based materials have been widely known as advanced non-noble electrocatalysts.16–19 In recent decades, metal oxides have raised great attention in the oxygen evolution reaction under alkaline conditions,20–22 which are considered as the most durable and active water oxidation catalysts.23,24 Among them, ruthenium (Ru) and iridium (Ir) oxides as the state-of-the-art OER catalysts are usually applied in commercial water oxidation to scaled-produce hydrogen, although they are rare in the earth crust.25–29 Meanwhile, it is reported that a serial of perovskite and spinel solids containing some transition metals (such as Ni and Co) also exhibit favorable OER activity in alkaline solution.30–33 Recently, amorphous metal oxides (such as Fe and Mg) have also been demonstrated to be excellent OER catalysts,34–37 and it is proved that metal oxides containing more than a single element could improve OER performance. Smith et al.35 and Liang et al.37 reported that the mixed-metal oxide films mixed with a-FeOx own an enhanced catalytic properties in OER and the catalytic activity of Co3O4 is enhanced by the presence of reduced graphene oxide. In such a case, the researches above inspire us to apply the multiple metal oxides that in situ grown on substrate to the study of water oxidation.
Copper–silver oxide nanowires (CuO–Ag2O NWs) were investigated for enhanced catalytic formaldehyde oxidation, which shown an excellent catalytic activity.38 Based on this material, we continued to study the water oxidation activity in alkaline solution. CuO–Ag2O NWs are simply fabricated by bathing CuAgZn substrate in Na2O2 aqueous solution and then annealing at 300 °C in the air for 3 h. Through electrochemical tests in 1 M KOH, it is found that Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag
Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn = 47%
Zn = 47%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28%
28%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25% by mass ratio shows the best catalytic performance for OER. At the same time, the relations between some electrochemical parameters and the compositions of copper, silver and zinc are also investigated systematically. Besides, CuO NPs, Ag2O NPs and CuO–Ag2O NPs are also prepared to prove the better catalytic performance of CuO–Ag2O NWs as OER catalyst and it displays the preferable electrochemical properties to OER in alkaline aqueous solution. In addition, the obtained CuO–Ag2O NWs also owns favorable catalytic stability, which can be a potential material in the application of water-splitting system.
25% by mass ratio shows the best catalytic performance for OER. At the same time, the relations between some electrochemical parameters and the compositions of copper, silver and zinc are also investigated systematically. Besides, CuO NPs, Ag2O NPs and CuO–Ag2O NPs are also prepared to prove the better catalytic performance of CuO–Ag2O NWs as OER catalyst and it displays the preferable electrochemical properties to OER in alkaline aqueous solution. In addition, the obtained CuO–Ag2O NWs also owns favorable catalytic stability, which can be a potential material in the application of water-splitting system.
2 Experimental
2.1. Preparation of CuO–Ag2O NWs
All the chemicals were of analytical purity and were used as received and diverse compositions of CuAgZn alloy were obtained following the description in the previous work.38 The preparation of CuO–Ag2O NWs was conducted as follows: fresh CuAgZn ternary alloy electrode was simply gained by removing the surface oxides layer on CuAgZn electrode with abrasive paper and washed in ethanol for 2 min. Then, 0.585 g Na2O2 was dispersed in 15 mL deionized aqueous solution. Subsequently, the prepared electrode was rapidly immersed in the Na2O2 aqueous solution at 40 °C in thermostat water bath for 40 min. After the bath, such electrode was inserted in muffle furnace at 300 °C for 3 h and then the prepared nanostructured oxides were cooled at room temperature. Each step of the preparation was followed by washing with distilled water twice and dried in air.2.2. Fabrication of CuO–Ag2O NPs
CuO–Ag2O was fabricated in the morphology of nanoparticles to compare with CuO–Ag2O NWs as followed. Smoothing and cleaning procedures were the same as the preparation of CuO–Ag2O NWs at the ternary alloy substrate. CuAgZn was then immersed in 1 M NaOH aqueous solution at 40 °C for 40 min, and the pre-treated substrate was annealed in muffle furnace at 300 °C for 3 h.39 Finally, the substrate covered with nanoparticles was cooled at room temperature. Similarly, distilled water washing is needed after each step.2.3. Preparation of Ag2O NPs and CuO NPs
Ag2O NPs were gained according to Sullivan et al.40 0.068 g AgNO3 was dissolved in 80 mL deionized aqueous solution and heated to 60 °C. 20 mL of 0.025 M NaOH aqueous solution was subsequently added drop-wise with a continuous stir until the solution turned to gray-yellow colloidal suspension. After 2 h, the prepared nanoparticles were then collected with ethanol washing in centrifuge for 3 times, and the solution was dried to get Ag2O nanoparticles. CuO NPs were fabricated based on the report of Sahooli et al.41 2.7 g Cu(Ac)2 and 2 mL CH3COOH were mixed with 600 mL ethylene glycol in the system of condensation. The solution was heated to 78 °C with a vigorous stir. 1 M NaOH ethylene glycol solution of 40 mL was then added dropwise until some black precipitation was generated. The CuO nanoparticles was cooled at room temperature, centrifuged with to remove impurities and washed with ethanol and deionizer water for several times.2.4. Characterization
X-Ray diffraction (XRD, DX-1000, China) and energy-dispersive X-ray spectrometry (EDS, EDAX Corp., USA) was used to analyze the compositions and elements of the nanostructures on the ternary alloy electrode, XRD was worked under the condition of low scanning rate (0.02° per second) and high power (6.0 kW) during XRD characterization. The field emission scanning electron microscopy (FESEM, Hitachi S4800, Japan) and high-resolution transmission electron microscopy (HRTEM, FEI Tecnai G2 F20 S, USA) were operated to image the morphology and size of the prepared materials.2.5. Electrochemical measurements
Electrochemical workstation (LK9805, Tianjin Lanlike Corp., China) was used to test the catalytic activity on water oxidation. Cyclic voltammetry (CV) scans and linear sweep voltammetry (LSV) were performed with a standard three-electrode configuration in 1 M potassium hydroxide (KOH) solution at a scan rate of 50 mV s−1 and 5 mV s−1, respectively. Pt sheet and Hg/HgO were used as the counter electrode and the reference electrode. The prepared multi-metal oxides in situ grown on CuAgZn substrate was directly used as the working electrode in the three-electrode system, with the diameter of 2 mm and effective test length of 10 mm. All the data were collected at a steady state after continuous scan. And the powder catalyst inks were consisted of 5 mg fabricated Ag2O NPs/CuO NPs, 140 μL isopropanol, 360 μL deionized water and 10 μL Nafion. Afterwards, drop ∼2 μL on the alloy electrode as the working electrode and dried at room temperature for overnight. All electrochemical potentials were corrected for uncompensated solution resistance (Ru, here Ru was fixed to 2 Ω) measured using the high frequency pulse function, and were given in the text relative to the reversible hydrogen electrode (vs. RHE):| ERHE = E + EHg/HgO + 0.059 pH − iRu | (3) |
Tafel plots were gained from LSV via an operation of log to the current density versus the potential, and the Tafel region began when the plot went to a liner rise. The Tafel slope was the relative slope of the Tafel curve with the range from the beginning of Tafel region to the edge of measured potential. The onset potential is defined the point where the linear Tafel region begins and it is deduced by the Tafel plot during the low current density region. The amount of dissolved oxygen was detected by a PASCO oxygen probe (USA). And the total mole of O2 was calculated according to the Henry Law. The electrochemical impedance spectrum (EIS) was recorded by the electrochemical workstation (Autolab PGSTAT 12 potentiostat/galvanostat, USA) with Pt counter electrode and Hg/HgO reference electrode.
3 Results and discussion
3.1. Characterization of the nanowires
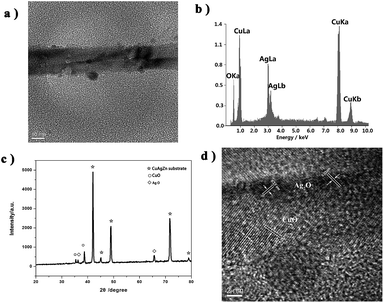
Preparation of CuO–Ag2O NWs is described in Fig. 1a. As we can see, the CuAgZn substrate is covered with lots of nanofibers (NFs) after bathed in Na2O2 aqueous solution, and the diameter of the fibers is ∼10 nm with the length of 100–200 nm (Fig. 1b), according to the published paper.38 These NFs are proved to be CuO and Ag2O. After the second preparation of annealed in 300 °C, the morphology of CuO–Ag2O is transferred to nanowires, and the length becomes longer to about 400–600 nm (Fig. 1c) with the diameter of those NWs increasing to ∼20 nm (Fig. 2a). X-Ray diffraction (XRD) and energy-dispersive X-ray spectrometry (EDS) are firstly utilized to identify the NWs, and the pattern of EDS indicates that there are three elements of Cu, Ag and O in the NWs corresponding to two oxides. Together with XRD analysis, the two types of oxides are verified as CuO and Ag2O (Fig. 2b and c). As showed in the pattern of high-resolution transmission electron microscopy (HRTEM), there are two kinds of lattice fringes in the NWs, and one lattice spacings of CuO are 0.23–0.25 nm, with another one of Ag2O 0.19–0.21 nm (Fig. 2d).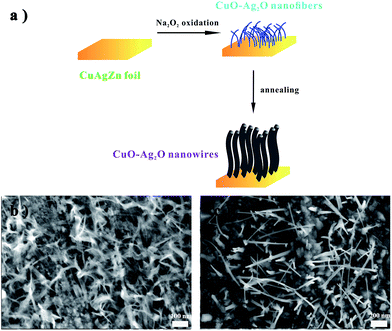 | ||
| Fig. 1 (a) Procedure of the preparation of CuO–Ag2O NWs on CuAgZn substrate and FESEM images of CuO–Ag2O NFs (b) and CuO–Ag2O NWs (c). | ||
In procedure of the fabrication of CuO–Ag2O NWs, zinc is firstly sacrificed in the alloy substrate to promote CuO and Ag2O to form crystalline nucleus and grow its crystal with a simple process as follows:
The Na2O2 dissolves in aqueous solution, which creates an alkaline condition with a lot of oxygen. Then zinc on the surface of the CuAgZn alloy substrate is firstly corroded, with some cavities and lattice defect created. In addition, Cu and Ag in the alloy are exposed to the oxygen-rich atmosphere. Coupled with the course of oxygen diffusion, the reaction of oxidation occurs to form CuO and Ag2O. With continuous oxidation, CuO and Ag2O gradually crystallize and shape NFs in the oxygen-rich alkaline aqueous solution. In the subsequent annealing at 300 °C for 3 h, the NFs continue growing and crystallizing. Finally the NWs are successfully formed from NFs.
3.2. Electrochemical properties of the CuO–Ag2O NWs
In order to test the catalytic properties of CuO–Ag2O NWs on the oxidation of the water, we conduct linear sweep voltammetry (LSV) on the prepared NWs. In Fig. 3a and b, we compare bare CuAgZn substrste with CuO–Ag2O NWs in 1 M KOH solution at a potential range of 0.2 V–1.2 V vs. Hg/HgO. The bare ternary alloy electrode without oxides layer on the surface exhibits an onset potential at 1.66 ± 0.01 V vs. RHE (onset potential: where the linear Tafel region begins) and a Tafel slope of 244 ± 9 mV dec−1 at the potential of 1.66 ± 0.01 V vs. RHE. It suggests that the bare alloy substrate hardly owns OER catalytic activity, which cannot affect the catalytic property of the NWs. To test whether our prepared CuO–Ag2O superior to the singe metallic oxide, flake-like copper and silver in the same preparation environment (CuO and Ag2O) are also prepared and still show unsatisfied result with large onset potential and high Tafel slope. Thus it deduces that CuO and Ag2O have very poor OER activity, which may be defined as inferior catalysts for OER. In Fig. 3a, though RuO2 shows a comparable lower onset potential, its over potential is inferior with the increasing of current density. Remarkably, CuO–Ag2O NWs have an improved catalytic activity and show a lower OER onset potential (∼1.62 ± 0.01 V vs. RHE) and a smaller Tafel slope (∼118 ± 4 mV dec−1). It indicates that CuO–Ag2O NWs have synergistic effect on OER catalytic activity between Ag2O and CuO and enable the two oxides to interact to accelerate the rate of catalyzing OER. And in Fig. 3a, it can be observed that CuO–Ag2O NWs possess a characteristic precatalytic oxidation peak centered at 1.55 ± 0.01 V vs. RHE, which may be attributed to the oxidation of a lower state Ag species formed during the initial sweep through the catalytic wave, since there is no oxidation peak at the LSV curve of CuO42 but that of Ag2O gets one. Such metal oxidation process might generate the active center with high valence, which could improve the performance.3.3. The effect of the compositions of CuAgZn substrate on OER activity
Other 16 groups of CuAgZn alloy electrodes of different compositions are prepared by the same preparation. Four typical kinds of alloy oxides with different proportions (CuAgZnOx) are contrasted in Fig. 4a. Among them, Cu20Ag60Zn20Ox gains the onset potential of 1.68 ± 0.01 V vs. RHE and a Tafel slope of 166 ± 6 mV dec−1, and its catalytic performance is not as good as Cu47g28Zn25Ox, Cu10Ag20Zn70Ox and Cu87Ag6Zn7Ox, which shows that Cu47Ag28Zn25 has the proper composition of copper, silver and zinc among the serial alloy. Thus Cu47g28Zn25Ox has a best good catalytic performance on water oxidation reaction in alkaline aqueous solution. | ||
| Fig. 4 (a) LSV curves of four kinds of alloy oxides with different compositions; (b) Tafel slopes and onset over potential (η vs. 1.23 V) of the four kinds of alloy oxides. | ||
Several relationships between the kinetic parameters and the ternary metal substrate combinations are shown in the trigonal coordinate plot apparently in Fig. 5. The Tafel slopes show an obvious effect by the amount of copper (Fig. 5a). In addition, when the amount of copper is the major content, the alloy oxides with higher concentrations of silver are found to produce higher Tafel slopes. And the Tafel slope is low when the composition is centered on Cu47Ag28Zn25, which can be seen in the red area in Fig. 5a; that is, oxides rich in silver but short of copper afford Tafel slopes over the 130–160 mV dec−1 range, while oxides with a comparably less silver produce slopes of 100–130 mV dec−1, especially at Cu47Ag28Zn25 of 118 ± 4 mV dec−1. Meanwhile, the relationship between potential at 30 mA cm−2 and the compositions of CuAgZn alloy is showed in Fig. 5b, which implies that the alloy containing equivalent amount of copper, silver and zinc after preparation have relatively low over potential to reach 30 mA cm−2, while too much copper or silver can make the over potential over 0.55 ± 0.01 V, which is seen in the blue area in Fig. 5b. And the detailed data are kept in Table 1, we also list the relations between the over potential when the current density is 20 mA cm−2 and 30 mA cm−2 with the amount of copper, silver and zinc in the alloy substrates in the table.
 | ||
| Fig. 5 A trigonal coordinate plot illustrating the relationship between the compositions of CuAgZn and the Tafel slopes (a), and η vs. 1.23 V when the current density was 30 mA cm−2 (b). | ||
| % Cu | % Ag | % Zn | Onset η (V) | Tafel slope (mV dec−1) | η@20 (mA cm−2) | η@30 (mA cm−2) |
|---|---|---|---|---|---|---|
| a Undetected; η@20 (mA cm−2) and η@30 (mA cm−2) have the same deviation standard as onset η corresponding to the specific alloy. | ||||||
| 8 | 52 | 40 | 0.44 ± 0.01 | 234 ± 5 | —a | 0.478 |
| 10 | 20 | 70 | 0.43 ± 0.02 | 145 ± 6 | 0.509 | 0.543 |
| 20 | 30 | 50 | 0.45 ± 0.01 | 253 ± 4 | —a | 0.491 |
| 20 | 60 | 20 | 0.42 ± 0.01 | 166 ± 7 | 0.557 | 0.601 |
| 31 | 7 | 62 | 0.42 ± 0.02 | 120 ± 3 | 0.465 | 0.487 |
| 37 | 41 | 22 | 0.42 ± 0.01 | 115 ± 6 | 0.467 | 0.490 |
| 41 | 16 | 43 | 0.41 ± 0.01 | 177 ± 5 | 0.534 | 0.575 |
| 42 | 32 | 26 | 0.44 ± 0.01 | 107 ± 9 | 0.509 | 0.529 |
| 45 | 9 | 46 | 0.39 ± 0.02 | 164 ± 10 | 0.512 | 0.553 |
| 47 | 28 | 25 | 0.38 ± 0.01 | 118 ± 4 | 0.448 | 0.475 |
| 49 | 43 | 8 | 0.41 ± 0.02 | 145 ± 5 | 0.458 | 0.480 |
| 50 | 50 | 0 | 0.41 ± 0.02 | 167 ± 7 | 0.571 | 0.634 |
| 60 | 5 | 35 | 0.40 ± 0.03 | 102 ± 8 | 0.465 | 0.484 |
| 87 | 6 | 7 | 0.40 ± 0.02 | 117 ± 10 | 0.485 | 0.507 |
| 90 | 10 | 0 | 0.39 ± 0.02 | 270 ± 11 | 0.504 | 0.617 |
| 0 | 100 | 0 | 0.50 ± 0.01 | 166 ± 8 | —a | 0.516 |
| 100 | 0 | 0 | 0.39 ± 0.01 | 168 ± 5 | 0.567 | 0.617 |
3.4. Characterization of CuO–Ag2O NPs
CuO–Ag2O NPs are utilized to be compared with NWs for OER catalytic performance. The Na2O2 dissolved in deionized water creates an environment of oxygen full in the aqueous solution, different to the NaOH alkaline aqueous solution without oxygen.According to its SEM pattern (Fig. 6a), the alloy substrate under this preparation is packed with lots of nanoparticles and the sizes range from ∼50 to 200 nm. According to the previously reported paper,39 these particles are identified to CuO–Ag2O NPs on the surface of the electrode. In Fig. 6d, with different morphology from nanowires, the CuO or Ag2O nanoparticles alone have obviously worse electrocatalytic activity on the oxidation of water than bimetallic CuO–Ag2O NWs and CuO–Ag2O NPs. The electrode modified with CuO and Ag2O achieves a higher Tafel slope of 320 ± 8 mV dec−1 and 210 ± 9 mV dec−1, meanwhile they also get a higher over potential, respectively. Similarly, CuO–Ag2O NPs obtained enhanced catalytic performance than CuO NPs and Ag2O NPs alone because of the synergetic coupling effects between two kinds of nanoparticles. And it is clear that CuO–Ag2O NPs achieve 10 mA cm−2 at the potential of 1.77 ± 0.01 V (Fig. 6b) and get the Tafel curve rise at 1.70 ± 0.01 V with a Tafel slope of 161 mV dec−1, as shown in Fig. 6c. Together with the CV curve in Fig. 6d, it is obvious that the OER performance of CuO–Ag2O NPs is also inferior to CuO–Ag2O NWs. That is because nanowire structure has high surface-to-volume ratios, more active sites exposal and minimum power consumption, which can make contribution to the water oxidation reaction to oxygen as effective electrocatalyst.43 In addition, the direct growth of catalyst on the substrate also constructs the self-stand nanowires shape increasing the interaction of the solution and electrode.
 | ||
| Fig. 6 (a) SEM pattern of CuO–Ag2O NPs; (b) and (c) LSV curves and Tafel curves of CuO–Ag2O NWs, CuO–Ag2O NPs, CuO NPs and Ag2O NPs; (d) CV curves of the four kinds of electrodes. | ||
3.5. Test on the electrochemical impedance and stability of catalyst
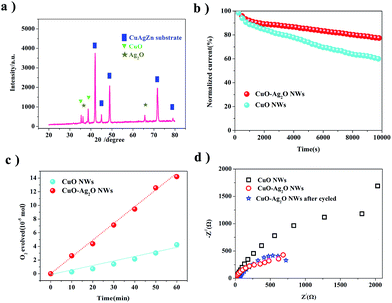
In 1.0 M KOH electrolyte, the prepared CuO–Ag2O NWs afford a comparably good electrocatalytic performance in OER.To get insight on the process of CuO–Ag2O NWs on OER, we firstly analyst the XRD pattern of our binary oxides NWs after 200 cycles. From Fig. 7a, we can judge that the CuO–Ag2O still exist on the CuAgZn substrate with the amount of the two kinds of oxides a little less. In addition, our prepared CuO–Ag2O NWs exhibit a relatively good durability in electrolysis test with the applied potential at 1.0 V vs. Hg/HgO, and these nanowires have little decay in OER activity over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s of continuous operation. In Fig. 7b, the normalized current of CuO–Ag2O NWs drops fast at the first 1000 s, and it comes to decrease slowly when the time reach 2000 s, with the steady decay rate in the whole test, the normalized current remains 80%. In Fig. 7c, Faradaic efficiency test of CuO NWs and CuO–Ag2O NWs is performed in 60 minutes, the curves shows that our binary oxides NWs obtain the ratio of oxygen released between measured value and theory value is close to 100%. We also conduct an electrochemical impedance spectrum (EIS) in 1.0 M KOH electrolyte (Fig. 7d). It reveals that CuO–Ag2O NWs as the OER catalyst have a smaller semicircle at a high frequency region than CuO, which indicates lower charge-transfer resistance (Rct) of CuO–Ag2O NWs in the system to pass electron more effective, since the content of Ag in the alloy substrate owns excellent electric conductivity. After cycled, the Ret has only slightly increased because of surface nanocrystals dissolution as well as aggregation,37 revealing the stable structure of the electrode. Thus our prepared binary metal oxides (CuO and Ag2O) have favorable good long-time durability in alkaline aqueous solution, which makes it promising for OER and other important catalytic reactions in alkaline solutions.
000 s of continuous operation. In Fig. 7b, the normalized current of CuO–Ag2O NWs drops fast at the first 1000 s, and it comes to decrease slowly when the time reach 2000 s, with the steady decay rate in the whole test, the normalized current remains 80%. In Fig. 7c, Faradaic efficiency test of CuO NWs and CuO–Ag2O NWs is performed in 60 minutes, the curves shows that our binary oxides NWs obtain the ratio of oxygen released between measured value and theory value is close to 100%. We also conduct an electrochemical impedance spectrum (EIS) in 1.0 M KOH electrolyte (Fig. 7d). It reveals that CuO–Ag2O NWs as the OER catalyst have a smaller semicircle at a high frequency region than CuO, which indicates lower charge-transfer resistance (Rct) of CuO–Ag2O NWs in the system to pass electron more effective, since the content of Ag in the alloy substrate owns excellent electric conductivity. After cycled, the Ret has only slightly increased because of surface nanocrystals dissolution as well as aggregation,37 revealing the stable structure of the electrode. Thus our prepared binary metal oxides (CuO and Ag2O) have favorable good long-time durability in alkaline aqueous solution, which makes it promising for OER and other important catalytic reactions in alkaline solutions.
4. Conclusion
In summary, through a simple and fast method, we have produced a kind of nanostructured binary metal oxides, which are proved to be CuO–Ag2O NWs, on the substrate of alloy electrode. Besides, the prepared metal oxides have comparable better catalytic performance on OER in alkaline aqueous solution than single metallic CuO and Ag2O. The best composition of Cu47Ag28Zn25Ox obtains an onset potential of 1.64 V vs. RHE and gains a Tafel slope of 118 mV dec−1. In addition to comparably good electron transportation efficiency, our CuO–Ag2O NWs catalyst has relatively good electrocatalytic stability, with retention of the normalized current to 80% in 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s. Therefore, the simple method to obtain multimetallic oxide nanocatalysts we used might provide a way to fabricate other nanostructures as efficient catalysts.
000 s. Therefore, the simple method to obtain multimetallic oxide nanocatalysts we used might provide a way to fabricate other nanostructures as efficient catalysts.
Acknowledgements
We gratefully acknowledge the financial support for this research by the “Scientific Exploration Project for Undergraduates” of Sichuan Province (no. 20130071) and Sichuan University (no. 20130391).Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- F. Cheng and J. Chen, Chem. Soc. Rev., 2012, 41, 2172–2192 RSC.
- H. B. Gray, Nat. Chem., 2009, 1, 112 CrossRef CAS.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- Y. Liang, Y. Li, H. Wang and H. Dai, J. Am. Chem. Soc., 2013, 135, 2013–2036 CrossRef CAS PubMed.
- Y.-C. Lu, Z. Xu, H. A. Gasteiger, S. Chen, K. Hamad-Schifferli and Y. Shao-Horn, J. Am. Chem. Soc., 2010, 132, 12170–12171 CrossRef CAS PubMed.
- R. D. Smith, M. S. Prevot, R. D. Fagan, Z. Zhang, P. A. Sedach, M. K. Siu, S. Trudel and C. P. Berlinguette, Science, 2013, 340, 60–63 CrossRef CAS PubMed.
- H. Wang and H. Dai, Chem. Soc. Rev., 2013, 42, 3088–3113 RSC.
- S. W. Lee, C. Carlton, M. Risch, Y. Surendranath, S. Chen, S. Furutsuki, A. Yamada, D. G. Nocera and Y. Shao-Horn, J. Am. Chem. Soc., 2012, 134, 16959–16962 CrossRef CAS PubMed.
- T. A. Betley, Q. Wu, T. Van Voorhis and D. G. Nocera, Inorg. Chem., 2008, 47, 1849–1861 CrossRef CAS PubMed.
- R. I. Cukier and D. G. Nocera, Annu. Rev. Phys. Chem., 1998, 49, 337–369 CrossRef CAS PubMed.
- S. Hammes-Schiffer, Acc. Chem. Res., 2009, 42, 1881–1889 CrossRef CAS PubMed.
- M. H. V. Huynh and T. J. Meyer, Chem. Rev., 2007, 107, 5004–5064 CrossRef CAS PubMed.
- E. Fabbri, A. Habereder, K. Waltar, R. Kotz and T. J. Schmidt, Catal. Sci. Technol., 2014, 4, 3800–3821 CAS.
- Z. Y. Jin, P. P. Li and D. Xiao, Sci. Rep., 2014, 4, 6712–6718 CrossRef PubMed.
- Z. Jin, P. Li, X. Huang, G. Zeng, Y. Jin, B. Zheng and D. Xiao, J. Mater. Chem. A, 2014, 2, 18593–18599 CAS.
- P. Li, Z. Jin and D. Xiao, J. Mater. Chem. A, 2014, 2, 18420–18427 CAS.
- H. Pan, Sci. Rep., 2014, 4, 5348–5353 Search PubMed.
- J. O. Bockris and T. Otagawa, J. Phys. Chem., 1983, 87, 2960–2971 CrossRef CAS.
- M. D. Merrill and R. C. Dougherty, J. Phys. Chem. C, 2008, 112, 3655–3666 CAS.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072–1075 CrossRef CAS PubMed.
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS PubMed.
- A. T. Marshall and R. G. Haverkamp, Electrochim. Acta, 2010, 55, 1978–1984 CrossRef CAS PubMed.
- J. D. Blakemore, N. D. Schley, D. Balcells, J. F. Hull, G. W. Olack, C. D. Incarvito, O. Eisenstein, G. W. Brudvig and R. H. Crabtree, J. Am. Chem. Soc., 2010, 132, 16017–16029 CrossRef CAS PubMed.
- J. D. Blakemore, N. D. Schley, G. W. Olack, C. D. Incarvito, G. W. Brudvig and R. H. Crabtree, Chem. Sci., 2011, 2, 94–98 RSC.
- J. F. Hull, D. Balcells, J. D. Blakemore, C. D. Incarvito, O. Eisenstein, G. W. Brudvig and R. H. Crabtree, J. Am. Chem. Soc., 2009, 131, 8730–8731 CrossRef CAS PubMed.
- T. Nakagawa, C. A. Beasley and R. W. Murray, J. Phys. Chem. C, 2009, 113, 12958–12961 CAS.
- T. Nakagawa, N. S. Bjorge and R. W. Murray, J. Am. Chem. Soc., 2009, 131, 15578–15579 CrossRef CAS PubMed.
- B. S. Brunschwig, M. H. Chou, C. Creutz, P. Ghosh and N. Sutin, J. Am. Chem. Soc., 1983, 105, 4832–4833 CrossRef CAS.
- M. Dincă, Y. Surendranath and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10337–10341 CrossRef PubMed.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- Y. Surendranath, M. Dincǎ and D. G. Nocera, J. Am. Chem. Soc., 2009, 131, 2615–2620 CrossRef CAS PubMed.
- M. W. Louie and A. T. Bell, J. Am. Chem. Soc., 2013, 135, 12329–12337 CrossRef CAS PubMed.
- R. D. Smith, M. S. Prevot, R. D. Fagan, S. Trudel and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135, 11580–11586 CrossRef CAS PubMed.
- I. Zaharieva, P. Chernev, M. Risch, K. Klingan, M. Kohlhoff, A. Fischer and H. Dau, Energy Environ. Sci., 2012, 5, 7081–7089 CAS.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed.
- Z. Jin, P. Li, G. Liu, B. Zheng, H. Yuan and D. Xiao, J. Mater. Chem. A, 2013, 1, 14736 CAS.
- Z. Jin, P. Li, B. Zheng, H. Yuan and D. Xiao, Anal. Methods, 2014, 6, 2215 RSC.
- K. T. Sullivan, C. Wu, N. W. Piekiel, K. Gaskell and M. R. Zachariah, Combust. Flame, 2013, 160, 438–446 CrossRef CAS PubMed.
- M. Sahooli, S. Sabbaghi and R. Saboori, Mater. Lett., 2012, 81, 169–172 CrossRef CAS PubMed.
- Z. Chen, A. R. Rathmell, S. Ye, A. R. Wilson and B. J. Wiley, Angew. Chem., Int. Ed. Engl., 2013, 52, 13708–13711 CrossRef CAS PubMed.
- N. S. Ramgir, Y. Yang and M. Zacharias, Small, 2010, 6, 1705–1722 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |