Hierarchical NiCo2O4 nanowire arrays on Ni foam as an anode for lithium-ion batteries
Abstract
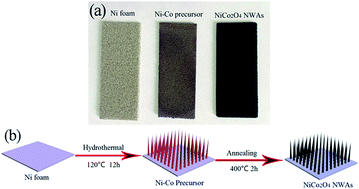
A facile two-step method is developed for large-scale growth of hierarchical mesoporous NiCo2O4 nanowire arrays on Ni foam with robust adhesion as a high performance electrode for lithium-ion batteries (LIBs). The as-prepared mesoporous NiCo2O4 nanowire arrays consist of numerous highly crystalline nanoparticles, the Ni foam supported NiCo2O4 nanowires promote fast electron and ion transport, and alleviate the volume change during the charge–discharge processes. When evaluated as a binder-free electrode for LIBs, the NiCo2O4 electrode exhibits a greatly enhanced charge capacity of 1520 mA h g−1 at a current density of 100 mA g−1. The mesoporous NiCo2O4 nanowire arrays exhibit great potential as an anode material for LIBs with the advantages of unique performance and facile preparation.

 Please wait while we load your content...
Please wait while we load your content...