Synthesis and characterization of aromatic poly(amides) based on 3,5-diamino-N-cyclopropylbenzamide
Abstract
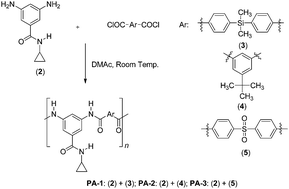
Three aromatic poly(amides) (PAs) were prepared, one of them containing a dimethyldiphenylsilane unit from a new aromatic diamine monomer with a bulky pendant polar group. All PAs were obtained in high yield and the inherent viscosities were in the range of 0.30 and 0.47 dL g−1. The obtained poly(amides) showed excellent solubility in a variety of aprotic polar organic solvents. PAs evidence thermal stability with thermal decomposition temperature (TDT10%) between 300–371 °C and the glass transition temperatures (Tg) values were high (between 215 and 250 °C). PAs containing silicon atom in the main chain captures the highest moles number of water per mole of repeating unit. Contact angles were also tested in order to know the hydrophilicity of the polymer films.

 Please wait while we load your content...
Please wait while we load your content...