Methylsulfone as a leaving group for synthesis of hyperbranched poly(arylene pyrimidine ether)s by nucleophilic aromatic substitution†
Yue Guan,
Chunbo Wang,
Daming Wang,
Guodong Dang,
Chunhai Chen,
Hongwei Zhou and
Xiaogang Zhao*
Alan G. MacDiarmid Institute of Jilin university, Changchun, 130012, PR China. E-mail: xiaogang@jlu.edu.cn
First published on 16th January 2015
Abstract
Using a novel leaving group, methylsulfone activated by pyrimidine, 4,6-dichloro-2-(methylsulfonyl)pyrimidine was used to synthesize two new hyperbranched poly(arylene pyrimidine ether)s with diphenol via a nucleophilic substitution polymerization.
In the past two decades, the macromolecules in three dimensions have been a subject of intensive research focusing on the control of structures and functions due to the unique characteristics of three-dimensional macromolecules and their high application potential in many areas.1 As important macromolecules, branched polymers, are interesting and versatile materials, and display unique properties, such as low solution and melt viscosities, high solubility and a high degree of functionality, compared to their linear analogs because of their globular structure and spherical shape.2,3 These features make such highly branched polymers attractive candidates for many material applications in coatings, supports for catalysts, drug and nanotechnology, and surface modification.4–8
Nucleophilic aromatic substitution reaction (SNAr) has been known as a very effective way for the formation of aromatic ether groups and utilized in the synthesis of many organic and polymeric compounds.9 The SNAr reaction generally requires a leaving group activated by an electron-withdrawing group such as sulfone, ketone and certain heterocycle.10 Typical leaving groups are fluorine, bromine, and nitro groups.9,11,12 Certain aromatic heterocyclic functions similarly, and reports of the use of these nonconventional activating groups in polymer-forming reactions have appeared recently. Poly(arylene ether)s and related polymers comprise a class of materials known as engineering thermoplastics which possess desirable properties including melt and solution processability, high Tg (glass transition temperature), and good mechanical properties.13–15 It is of obvious interest to extend this synthesis of hyperbranched poly(arylene ethers) to heterocycle-activated systems.
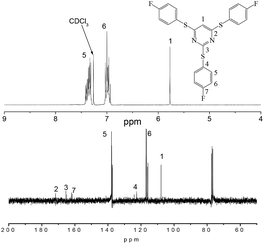
In this work, we presented a new leaving group (–SO2CH3) activated by pyrimidine. The reactivity of the monomer, 4,6-dichloro-2-(methylsulfonyl)pyrimidine (DMP), in a nucleophilic aromatic substitution mechanism was estimated using 13C NMR spectroscopy (Fig. 1). The data support the methylsulfone as a leaving group activated by pyrimidine ring for substitution by phenoxide nucleophiles. Moreover, Baiazitov16 has reported the calculated partial atomic charges on the chlorine- and sulfur-bound carbon atoms in pyrimidine that shows essentially the same activity of methylsulfone and chloride (Fig. 1). According to the results, we presented 4,6-dichloro-2-(methylsulfonyl)pyrimidine as a “BB′2” monomer reacted with 4,4′-thiobisbenzenethiol and 4,4′-thiodiphenol (A2) to synthesize two highly hyperbranched poly(arylene ether)s (HB-PAEs). The structure of synthesized hyperbranched polymers has been characterized by NMR and FTIR, while gel permeation chromatography (GPC), DSC, TGA and UV-Vis were used to investigate the properties.
The model compound has been synthesized by the reaction of 4,6-dichloro-2-(methylsulfonyl)pyrimidine and 4-fluorothiophenol (Scheme 1). The model compound was synthesized at room temperature to give a high yield (95%) which indicated the methylsulfonyl high reactivity as a leaving group activated by pyrimidine ring for substitution by thiophenoxide nucleophiles. The resulting model compound was characterized by 1H NMR, 13C NMR and HPLC-MS. 1H NMR spectra (Fig. 2) of the model compound illustrates that the proton of the pyrimidine group appears at around δ 5.77 as a singlet, and the two protons of benzene appear at δ 7.36 and 7.02, respectively. In 13C NMR spectra (Fig. 2), the carbon 13 atoms in model compound show 7 signals, which resonate in the regions of 107–171 ppm. All the spectroscopic data obtained agrees with the expected structures. The above model reaction results reveal that the displacement of phenoxide and the methylsulfonyl group of 4,6-dichloro-2-(methylsulfonyl)pyrimidine is feasible at proper reaction conditions.
On the basis of the results of the model reaction, synthesis of new HB-PAEs containing pyrimidine units was attempted by nucleophilic aromatic substitution of 4,6-dichloro-2-(methylsulfonyl)pyrimidine (DMP) with 4,4′-thiobisbenzenethiol and 4,4′-thiodiphenol, as shown in Scheme 2. The polymerization with DMP and 4,4′-thiobisbenzenethiol was carried out via the one-pot method at room temperature for 12 h to give off-white polymer. Monitoring by GPC (Fig. S5†), the weight-average molecular weight of HB-PAE-1 for 1, 3, 6 and 12 h was 1.88, 2.46, 2.82 and 3.06 × 104, respectively. The results illustrate that the reaction carried out fast in 3 hours, and after that the reaction slowed down. Though the real molecular weight of the polymer may be even larger than the value estimated by GPC because dendritic macromolecules generally have smaller size than linear polymers with the same molecular weight and can hardly be expanded in solution.17 It is feasible that the GPC data with the same polystyrene standards were used to analyse the kinetics of the polymerization.
The polymerization with DMP and 4,4′-thiodiphenol was also carried out via the one-pot method. However, the high molecular weight polymer was not obtained at room temperature (∼25 °C). The result may be due to the weaker nucleophilic of phenoxide than that of thiophenoxide. After trying different reaction temperature, DMP can react with 4,4′-thiodiphenol at 55 °C to give high molecular weight polymer.
The degree of branching (DB) is an important characteristic often used to reveal the structure of hyperbranched polymers. A combination technique of model compound studies and 1H NMR spectroscopy (Fig. S1†) has been used to quantify the different subunits appearing in the hyperbranched polymer and subsequently determine its DB, and the results were summarized in Table 1.
The structures of the synthesized polymers were confirmed by 1H NMR and FTIR. As seen from Fig. 3, the FTIR spectra of HB-PAE-1 and HB-PAE-2 showed minor absorption peaks corresponding to methyl at around 3100 cm−1, and the 1H NMR (Fig. S1†) spectra of HB-PAE-1 and HB-PAE-2 also showed the peak, which suggested the methylsulfone did not completed react.
From Table 1, the calculated percentages of the conversion of methylsulfone were 92% and 94% for HB-PAE-1 and HB-PAE-2, respectively.
The characterization data and the thermal property of HB-PAEs are listed in Table S1.† HB-PAE-1 and HB-PAE-2, with inherent viscosity of 0.52 and 0.49 dL g−1, respectively, showed good solubility in common organic solvents, such as acetone, toluene, THF, CH2Cl2, and CHCl3 as well as polar aprotic solvents (DMF, DMAc, and NMP). The glass transition temperature (Tg) of HB-PAE-1 and HB-PAE-2 measured by DSC was 193 and 191 °C, respectively. The 5% weight loss temperature of HB-PAE-1 and HB-PAE-2 measured by TGA was 305 and 399 °C under nitrogen.
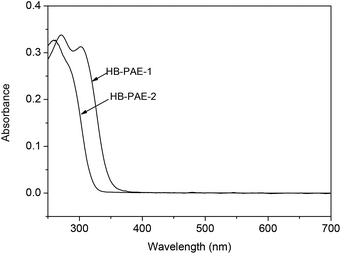
UV-vis absorption spectra (Fig. 4) in CHCl3 solution at room temperature showed two absorption peaks at 270 and 301 nm of HB-PAE-1 and only one absorption peak at 262 nm of HB-PAE-2. The λcut-off values of HB-PAE-1 and HB-PAE-2 films were 335 and 273 nm, respectively. The transmittance (%) at 450 nm of HB-PAE-1 and HB-PAE-2 films was 77% and 99%, respectively. The in-plane (nTE) and out-of-plane (nTM) refractive indices of the HB-PAE-1 at 633 nm are 1.7307 and 1.7218, respectively. The nav value of the polymer at 633 nm is 1.7277. The high refractive index of the polymer is obviously attributed to the introduction of a sulfur atom and pyrimidine unit into the polymer.18 Moreover, thioether linkages in the molecular chain of the polymer endow with the low Δn of 0.0089.
Conclusions
Methylsulfone was first employed as a leaving group to prepare hyperbranched poly(arylene ether)s by nucleophilic aromatic substitution. Two novel hyperbranched Poly(arylene pyrimidine ether)s were synthesized successfully using 4,4′-thiobisbenzenethiol and 4,4-thiodiphenol with 4,6-dichloro-2-(methylsulfonyl)pyrimidine under proper conditions utilizing methylsulfone as a new leaving group activated by pyrimidine via a simple nucleophilic substitution polymerization. The synthetic methodology described here provides a novel leaving group for the facile preparation of 3-dimensional macromolecules with a branched aromatic ether backbone containing pyrimidine units in the main chain, which are expected to find numerous applications, especially as an optical material.References
- Y. J. Kim, M. Kakimoto and S. Y. Kim, Macromolecules, 2006, 39, 7190–7192 CrossRef CAS.
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29, 183–275 CrossRef CAS PubMed.
- B. Voit, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 2679–2699 CrossRef CAS.
- L. Y. Qiu and Y. H. Bae, Pharm. Res., 2006, 23, 1–30 CrossRef CAS PubMed.
- T. H. Mourey, S. R. Turner, M. Rubinstein, J. M. J. Frechet, C. J. Hawker and K. L. Wooley, Macromolecules, 1992, 25, 2401–2406 CrossRef CAS.
- C. J. Hawker, P. J. Farrington, M. E. Mackay, K. L. Wooley and J. M. J. Frechet, J. Am. Chem. Soc., 1995, 117, 4409–4410 CrossRef CAS.
- D. Konkolewicz, Aust. J. Chem., 2009, 62, 823–829 CrossRef CAS.
- B. Liu, A. Kazlauciunas, J. T. Guthrie and S. Perrier, Macromolecules, 2005, 38, 2131–2136 CrossRef CAS.
- Y. J. Kim, I. S. Chung and S. Y. Kim, Macromolecules, 2003, 36, 3809–3811 CrossRef CAS.
- A. Ghosh, S. Banerjee, H. Komber, A. Lederer, L. Haussler and B. Voit, Macromolecules, 2010, 43, 2846–2854 CrossRef CAS.
- J. L. Hedrick, C. J. Hawker, R. D. Miller, R. Twieg, S. A. Srinivasan and M. Trollsas, Macromolecules, 1997, 30, 7607–7610 CrossRef CAS.
- M. H. Sun, J. li, B. S. Li, Y. Q. Fu and Z. S. Bo, Macromolecules, 2005, 38, 2651–2658 CrossRef CAS.
- R. J. Cotter, Engineering Plastics: A Handbook of Polyaryl ethers, Gordon and Breach Publishers, Amsterdam, 1995 Search PubMed.
- Y. Nakagawa, Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, Polym. Chem., 2012, 3, 2531–2536 RSC.
- Y. Nakagawa, Y. Suzuki, T. Higashihara, S. Ando and M. Ueda, Macromolecules, 2011, 44, 9180–9186 CrossRef CAS.
- R. Baiazitov, W. Du, C. S. Lee, S. H. wang, N. G. Almstead and Y. C. Moon, Synthesis, 2013, 45, 1764–1784 CrossRef CAS PubMed.
- J. Shen, Y. Zhang, W. Q. Chen, W. H. Wang, Z. S. Xu, K.-W. K. Yeung and C. F. Yi, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2425–2437 CrossRef CAS.
- N. H. You, T. Higashihara, Y. Oishi, S. Ando and M. Ueda, Macromolecules, 2010, 43, 4613–4615 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00634a |
| This journal is © The Royal Society of Chemistry 2015 |